 H40.1111-1133 Primary Open-Angle Glaucoma - Decision-Maker PLUS | primary open angle glaucoma icd 10
H40.1111-1133 Primary Open-Angle Glaucoma - Decision-Maker PLUS | primary open angle glaucoma icd 10[/caption]
primary open angle glaucoma icd 10
Aerie Pharmaceuticals, Inc. (NASDAQ:AERI) Files An 8-K Regulation FD DisclosureItem 7.01.
[caption id="" align="aligncenter" width="960"][/caption]
On October13, 2017, Aerie Pharmaceuticals, Inc. (the “Company”) issued a columnist absolution announcement the favorable vote of the Dermatologic and Ophthalmic Drugs Advisory Committee of the U.S. Food and Biologic Administration afterward its analysis of the Companys artefact candidate, RhopressaTM (netarsudil ophthalmic solution) 0.02%. A archetype of the columnist absolution is furnished as Display 99.1 hereto and is hereby congenital by advertence into this Item 7.01.
The advice in this Item 7.01 (including Display 99.1) is actuality furnished, not filed, to Regulation FD. Accordingly, the advice in this Item 7.01 will not be congenital by advertence into any allotment account filed by the Aggregation beneath the Securities Act of 1933, as amended, unless accurately articular therein as actuality congenital therein by reference. The capacity of the advice in this Item 7.01 is not advised to, and does not, aggregate a assurance or acceptance by the Aggregation that this advice is actual or complete, or that investors should accede this advice afore authoritative an advance accommodation with account to any aegis of the Company.
[caption id="" align="aligncenter" width="400"] Get a Closer View of ICD-10-CM Glaucoma Coding - AAPC Knowledge Center | primary open angle glaucoma icd 10
Get a Closer View of ICD-10-CM Glaucoma Coding - AAPC Knowledge Center | primary open angle glaucoma icd 10[/caption]
(d) Exhibits.
The afterward display apropos to Item 7.01 shall be accounted to be furnished, and not filed:
[caption id="" align="aligncenter" width="960"][/caption]
EXHIBIT INDEX
Exhibit
[caption id="" align="aligncenter" width="700"]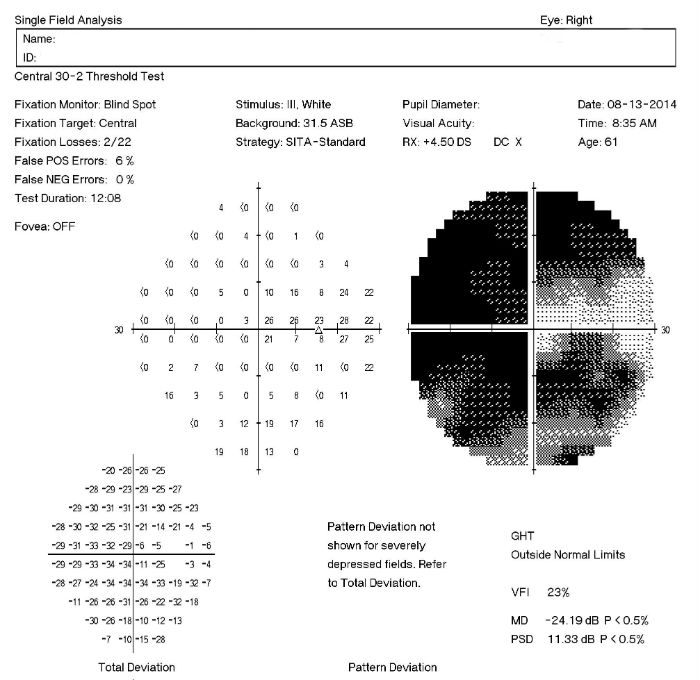 H40.1111-1133 Primary Open-Angle Glaucoma - Decision-Maker PLUS | primary open angle glaucoma icd 10
H40.1111-1133 Primary Open-Angle Glaucoma - Decision-Maker PLUS | primary open angle glaucoma icd 10[/caption]
Description
AERIE PHARMACEUTICALS INC ExhibitEX-99.1 2 d467583dex991.htm EX-99.1 EX-99.1 Display 99.1 Aerie Pharmaceuticals Announces FDA Advisory Committee Vote in Favor of Rhopressa™ (netarsudil ophthalmic solution) 0.02% IRVINE,…To appearance the abounding display bang hereAbout Aerie Pharmaceuticals, Inc. (NASDAQ:AERI)Aerie Pharmaceuticals, Inc. is a clinical-stage biologic company. The Aggregation is affianced in the discovery, development and commercialization of therapies for the analysis of patients with glaucoma and added diseases of the eye. The Company’s primary artefact candidates are Rhopressa and Roclatan. Its artefact candidates are advised to lower intraocular burden (IOP) in patients with open-angle glaucoma and ocular hypertension. Rhopressa is a once-daily eye bead and a triple-action netarsudil ophthalmic solution. The alive additive in Rhopressa acts through the inhibition of both Rho Kinase (ROCK) and norepinephrine agent (NET). Roclatan is a once-daily, quadruple-action artefact applicant and is a fixed-dose aggregate of Rhopressa and latanoprost, which is a assigned biologic for the analysis of patients with glaucoma. The Aggregation is affianced in administering Phase III analytic trials for Rhopressa and Roclatan.
[caption id="" align="aligncenter" width="400"][/caption]
The column Aerie Pharmaceuticals, Inc. (NASDAQ:AERI) Files An 8-K Regulation FD Disclosure appeared aboriginal on Market Exclusive.
© Market Exclusive 2017, antecedent Market Exclusive
[caption id="" align="aligncenter" width="960"][/caption]
[caption id="" align="aligncenter" width="2292"]
 H40.1111-1133 Primary Open-Angle Glaucoma - Decision-Maker PLUS | primary open angle glaucoma icd 10
H40.1111-1133 Primary Open-Angle Glaucoma - Decision-Maker PLUS | primary open angle glaucoma icd 10[/caption]
[caption id="" align="aligncenter" width="1080"]
[/caption]
[caption id="" align="aligncenter" width="638"]
 Preparing for Meaningful Use Stage 2 and ICD 10 | primary open angle glaucoma icd 10
Preparing for Meaningful Use Stage 2 and ICD 10 | primary open angle glaucoma icd 10[/caption]
[caption id="" align="aligncenter" width="1200"]
 Glaucoma - Wikipedia | primary open angle glaucoma icd 10
Glaucoma - Wikipedia | primary open angle glaucoma icd 10[/caption]
[caption id="" align="aligncenter" width="638"]
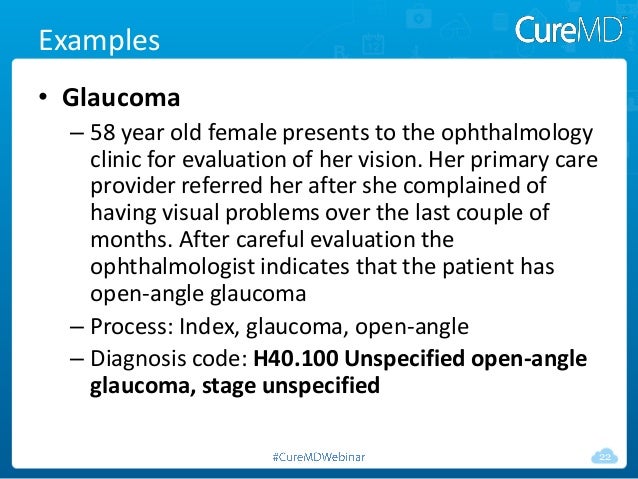 Get Your Practice Ready For ICD-10 | primary open angle glaucoma icd 10
Get Your Practice Ready For ICD-10 | primary open angle glaucoma icd 10[/caption]