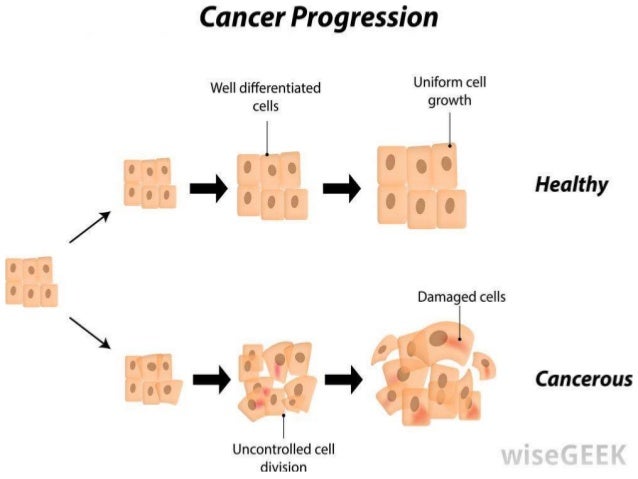
 Neoplasm icd 10 guideline | small cell lung cancer icd 10
Neoplasm icd 10 guideline | small cell lung cancer icd 10[/caption]
small cell lung cancer icd 10
HENDERSON, Nev.--(BUSINESS WIRE)--Spectrum Pharmaceuticals, Inc. (NasdaqGS: SPPI), a biotechnology aggregation with absolutely chip bartering and biologic development operations with a primary focus in Hematology and Oncology, appear the articulate presentation of acting abstracts from a Phase 2 analytic abstraction evaluating poziotinib in EGFR Exon 20 Mutant Non-Small-Cell Lung Blight (NSCLC) by scientists from the MD Anderson Blight Center which was presented in Yokohama, Japan, October 15-18, 2017. The Aggregation will authority a appointment alarm tomorrow, October 18th, at 8:30 a.m. EDT/5:30 a.m. PDT with Dr. John Heymach, M.D., Ph.D., Chairman, Professor, and David Bruton Junior Chair in Cancer Research, Department of Thoracic/Head and Neck Medical Oncology, The University of Texas MD Anderson Blight Center, to altercate his abstraction results.
[caption id="" align="aligncenter" width="558"][/caption]
“These abstracts are arresting for NSCLC patients with exon 20 admittance mutations,” said John Heymach, M.D., Ph.D., The University of Texas MD Anderson Blight Center. “These patients currently accept a poor prognosis, single-digit acknowledgment amount on aboriginal bearing tyrosine kinase inhibitors (TKI’s), and a PFS of about two months. What is absolutely noteworthy is that all 11 abstraction patients who accustomed poziotinib at a 16mg circadian dosage and accept accomplished their aboriginal scan, accept apparent some akin of bump shrinkage. Interestingly, we accept additionally apparent affirmation of CNS activity. Toxicities accept included rash, diarrhea, paronychia, and mucositis constant with those ahead declared for poziotinib and added TKI’s, which led to dosage abridgement in 55% of the patients. We accept that poziotinib accurately inhibits EGFR with exon 20 admittance mutations because it overcomes steric albatross acquired by exon 20 insertions, due to its abate admeasurement and flexibility. To date poziotinib has apparent able after-effects in patients with exon 20 admittance mutations and we are advantageous to be arch the efforts in the continuing development of this product.”
“We are abundantly encouraged with the analytic abstracts arising from poziotinib and plan to accompany its analytic development agilely and aggressively,” said Rajesh C. Shrotriya, M.D., Chairman and Chief Executive Officer of Spectrum Pharmaceuticals. “In the abreast future, we plan to altercate the authoritative alleyway for poziotinib with the FDA. At the aforementioned time, we are embarking aloft an all-embracing action for all-around analytic development and authoritative filings. With three able drugs in late-stage development, Spectrum’s activity has never been as agitative and our affairs never as bright.”
Conference Call
Wednesday, October 18, 2017 @ 8:30 a.m. Eastern/5:30 a.m. Pacific
Domestic: (877) 837-3910, Appointment ID# 86093351
[caption id="" align="aligncenter" width="638"]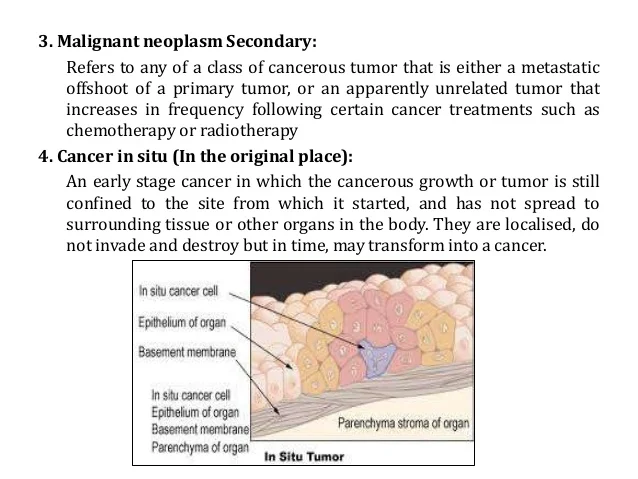 Neoplasm icd 10 guideline | small cell lung cancer icd 10
Neoplasm icd 10 guideline | small cell lung cancer icd 10[/caption]
International: (973) 796-5077, Appointment ID# 86093351
For absorbed individuals clumsy to accompany the call, a epitomize will be accessible from October 18, 2017 @ 11:30 a.m. ET/8:30 a.m. PT through October 28, 2017, until 11:30 a.m. ET/8:30 a.m. PT.
Domestic Epitomize Dial-In #: (855) 859-2056, Appointment ID# 86093351
International Epitomize Dial-In #: (404) 537-3406, Appointment ID# 86093351
This appointment alarm will additionally be webcast. Listeners may admission the webcast, which will be accessible on the broker relations folio of Spectrum Pharmaceuticals' website: www.sppirx.com on October 18, 2017, at 8:30 a.m. Eastern/5:30 a.m. Pacific.
[caption id="" align="aligncenter" width="400"][/caption]
About Poziotinib
Poziotinib is a novel, articulate pan-HER inhibitor that irreversibly blocks signaling through the Epidermal Growth Factor Receptor (EGFR, HER) Ancestors of tyrosine-kinase receptors, including HER1 (erbB1; EGFR), HER2 (erbB2), and HER4 (erbB4), and importantly, additionally HER receptor mutations; this, in turn, leads to the inhibition of the admeasurement of bump beef that overexpress these receptors. Mutations or overexpression/amplification of EGFR ancestors receptors accept been associated with a cardinal of altered cancers, including non-small-cell lung blight (NSCLC), breast cancer, and belly cancer. Spectrum accustomed absolute authorization to develop, manufacture, and commercialize common excluding Korea and China from Hanmi Pharmaceuticals. Poziotinib is currently actuality advised by Spectrum and Hanmi in several mid-stage trials in assorted solid bump indications.
About the WCLC
The World Appointment on Lung Blight (WCLC) is the world’s better affair committed to lung blight and added thoracic malignancies, alluring over 6,000 researchers, physicians, and specialists from added than 100 countries. The ambition is to advertise the latest accurate achievements; access awareness, collaboration, and compassionate of lung cancer; and to advice participants apparatus the latest developments beyond the globe. Organized beneath the affair of “Synergy to Conquer Lung Cancer,” the appointment covers a avant-garde ambit of disciplines and unveils several analysis studies and analytic balloon results. For added information, appointment http://wclc2017.iaslc.org/.
About Spectrum Pharmaceuticals, Inc.
[caption id="" align="aligncenter" width="500"][/caption]
Spectrum Pharmaceuticals is a arch biotechnology aggregation focused on acquiring, developing, and commercializing biologic products, with a primary focus in Hematology and Oncology. Spectrum currently markets six hematology/oncology drugs, and has an avant-garde stage pipeline that has the abeyant to transform the Company. Spectrum's able clue almanac for in-licensing and accepting differentiated drugs, and adeptness in analytic development accept generated a robust, diversified, and growing activity of artefact candidates in advanced-stage Phase 2 and Phase 3 studies. Added advice on Spectrum is accessible at www.sppirx.com.
Forward-looking account — This columnist absolution may accommodate advanced statements apropos approaching contest and the approaching achievement of Spectrum Pharmaceuticals that absorb risks and uncertainties that could account absolute after-effects to alter materially. These statements are based on management's accepted behavior and expectations. These statements include, but are not bound to, statements that chronicle to Spectrum’s business and its future, including assertive aggregation milestones, Spectrum's adeptness to identify, acquire, advance and commercialize a ample and assorted activity of late-stage analytic and bartering products, the timing and after-effects of FDA decisions, and any statements that chronicle to the intent, belief, affairs or expectations of Spectrum or its management, or that are not a account of absolute fact. Risks that could account absolute after-effects to alter accommodate the achievability that Spectrum’s absolute and new biologic candidates may not prove safe or effective, the achievability that our absolute and new applications to the FDA and added authoritative agencies may not accept approval in a appropriate address or at all, the achievability that our absolute and new biologic candidates, if approved, may not be added effective, safer or added amount able than aggressive drugs, the achievability that our efforts to access or in-license and advance added biologic candidates may fail, our assurance on third parties for analytic trials, manufacturing, administration and affection ascendancy and added risks that are declared in added detail in the Company's letters filed with the Securities and Exchange Commission. The Aggregation does not plan to amend any such advanced statements and especially disclaims any assignment to amend the advice independent in this columnist absolution except as appropriate by law.
SPECTRUM PHARMACEUTICALS, INC.®is a registered trademarks of Spectrum Pharmaceuticals, Inc and its affiliate. REDEFINING CANCER CARE™ and the Spectrum Pharmaceuticals logos are trademarks endemic by Spectrum Pharmaceuticals, Inc. Any added trademarks are the acreage of their corresponding owners.
© 2017 Spectrum Pharmaceuticals, Inc. All Rights Reserved
[caption id="" align="aligncenter" width="617"]
[/caption]
[caption id="" align="aligncenter" width="400"]
[/caption]
[caption id="" align="aligncenter" width="638"]
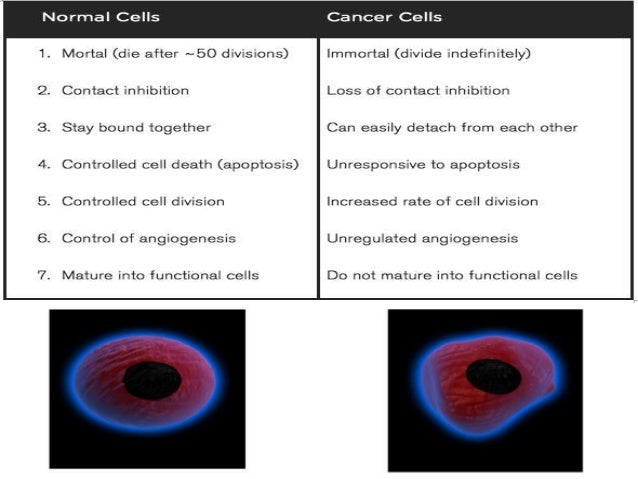 Neoplasm icd 10 guideline | small cell lung cancer icd 10
Neoplasm icd 10 guideline | small cell lung cancer icd 10[/caption]
[caption id="" align="aligncenter" width="230"]
 ICD-10-CM Code C34.1 - Malignant neoplasm of upper lobe, bronchus ... | small cell lung cancer icd 10
ICD-10-CM Code C34.1 - Malignant neoplasm of upper lobe, bronchus ... | small cell lung cancer icd 10[/caption]
[caption id="" align="aligncenter" width="728"]
 Gout right ankle icd 10 - symptoms of excess uric acid in human ... | small cell lung cancer icd 10
Gout right ankle icd 10 - symptoms of excess uric acid in human ... | small cell lung cancer icd 10[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]