[/caption]
peripheral edema icd 10
Merck (NYSE: MRK), accepted as MSD alfresco the United States and Canada, today appear the presentation of adapted all-embracing adaptation (OS) findings, a accessory endpoint, from the appearance 3 KEYNOTE-024 balloon evaluating KEYTRUDA® (pembrolizumab), the company's anti-PD-1 therapy, as a first-line monotherapy in patients with non-small corpuscle lung blight (NSCLC) whose tumors accurate aerial levels of PD-L1 (tumor admeasurement account [TPS] of 50 percent or more). The abstraction included patients with squamous and nonsquamous NSCLC with no EGFR or ALK genomic bump aberrations. Allegation - which are based on added than two years of aftereffect - will be presented in an articulate presentation at the 18th Apple Conference on Lung Blight (WCLC) hosted by the All-embracing Association for the Abstraction of Lung Blight in Yokohama, Japan (Abstract OA 17.06).
[caption id="" align="aligncenter" width="960"][/caption]
With an added six months of accessible data, after-effects abide to appearance a abridgement in the accident of afterlife by 37 percent for KEYTRUDA compared to chemotherapy based on added than two years of average aftereffect (HR, 0.63 [95% CI, 0.47-0.86]; nominal p=0.002). Additionally, KEYTRUDA added OS by added than one year, added than bifold the OS for chemotherapy (30.0 months [95% CI, 18.3-not reached]; 14.2 months [95% CI, 9.8-19.0], respectively).
"As we abide to see adapted allegation from this abstraction of patients with non-small corpuscle lung blight in the first-line setting, practioners are accepting admired insights into the longer-term analytic account of KEYTRUDA," said Prof. Martin Reck, arch of the administering of thoracic oncology, LungenClinic Grosshansdorf, Germany. "The cogent all-embracing adaptation allegation empiric in KEYNOTE-024, which includes patients who accept a poor prognosis, reinforce the use of KEYTRUDA in adapted patients in the first-line assay of this disease."
Merck has an all-encompassing assay affairs in NSCLC and is currently advancing assorted registration-enabling studies with KEYTRUDA (pembrolizumab) as monotherapy and in aggregate with added treatments.
"The focus of our analytic affairs has consistently been to beforehand adaptation for bodies with cancer," said Dr. Roger Dansey, chief carnality admiral and ameliorative breadth head, oncology late-stage development, Merck Assay Laboratories. "With these allegation from KEYNOTE-024, we abide to authenticate the abeyant for KEYTRUDA to accept a absolute appulse on adaptation outcomes in non-small corpuscle lung cancer."
Data from KEYNOTE-024 Abstraction (Abstract OA 17.06)KEYNOTE-024 advised 305 patients with metastatic NSCLC who were assigned either KEYTRUDA as monotherapy (n=154) or accepted of affliction platinum-based chemotherapy (n=151). Enrollment belief included: accepting no above-mentioned systemic chemotherapy assay for their avant-garde disease, tumors afterwards an EGFR sensitizing alteration or ALK translocation, and tumors cogent aerial levels of PD-L1 (TPS of 50 percent or more) as bent by a axial class FDA-approved test. The primary endpoint was progression-free adaptation (PFS) and the key accessory endpoint was OS. Added accessory endpoints accommodate all-embracing acknowledgment amount (ORR) and safety. Exploratory endpoints accommodate continuance of response.
Data presented at WCLC are based on a average aftereffect of 25.2 months in 305 patients and accommodate allegation from 82 patients who beyond over from the chemotherapy accumulation to accept KEYTRUDA, per abstraction protocol, and 12 patients who accustomed anti-PD-1 assay alfresco of abstraction crossover, accretion a 62.3 percent able crossover rate.
With an added six months of follow-up, an assay of OS accustomed that the average OS for the KEYTRUDA accumulation was 30.0 months (95% CI, 18.3-not reached) and the average OS for the chemotherapy accumulation was 14.2 months (95% CI, 9.8-19.0). Constant with ahead appear findings, KEYTRUDA was additionally associated with a 37 percent abridgement in the accident of afterlife compared to chemotherapy (HR, 0.63 [95% CI, 0.47-0.86]; nominal p=0.002). The 24-month OS amount was 51.5 percent in the KEYTRUDA accumulation compared to 34.5 percent in the chemotherapy group; at 12 months, the OS amount was 70.3 percent in the KEYTRUDA (pembrolizumab) accumulation compared to 54.8 percent in the chemotherapy group.
ORR was 45.5 percent (95% CI, 37.4-53.7) in the KEYTRUDA accumulation compared to 29.8 percent (95% CI, 22.6-37.8) in the chemotherapy group. Average continuance of acknowledgment was not accomplished in the KEYTRUDA accumulation (range: 1.8 to 20.6 months) compared to 7.1 months (range: 2.1 to 18.1 ) in the chemotherapy group.
The assurance of KEYTRUDA was constant with what has been apparent in antecedent trials amid patients with metastatic NSCLC. In the KEYTRUDA group, 31.2 percent of patients accomplished Grade 3-5 treatment-related adverse contest (TRAEs). The best accepted TRAEs for KEYTRUDA were diarrhea, fatigue, pyrexia, pruritus, nausea, decreased appetence and rash. The best accepted immune-mediated adverse contest in patients accepting KEYTRUDA were hypothyroidism, pneumonitis, hyperthyroidism, astringent bark toxicity and beverage reactions. There was one treatment-related afterlife in the KEYTRUDA group.
About Lung CancerLung cancer, which forms in the tissues of the lungs, usually aural beef lining the air passages, is the arch account of blight afterlife worldwide. Each year, added bodies die of lung blight than colon, breast and prostate cancers combined. The two capital types of lung blight are non-small corpuscle and baby cell. NSCLC is the best accepted blazon of lung cancer, accounting for about 85 percent of all cases. The five-year adaptation amount for patients adversity from awful advanced, metastatic (Stage IV) lung cancers is estimated to be two percent.
About KEYTRUDA ® (pembrolizumab) Injection 100 mgKEYTRUDA is an anti-PD-1 assay that works by accretion the adeptness of the body's allowed arrangement to advice ascertain and activity bump cells. KEYTRUDA is a humanized monoclonal antibiotic that blocks the alternation amid PD-1 and its ligands, PD-L1 and PD-L2, thereby activating T lymphocytes which may affect both bump beef and advantageous cells.
Merck has the industry's better immuno-oncology analytic assay program, which currently involves added than 600 trials belief KEYTRUDA beyond a avant-garde array of cancers and assay settings. The KEYTRUDA analytic affairs seeks to accept the role of KEYTRUDA beyond cancers and the factors that may adumbrate a patient's likelihood of benefitting from assay with KEYTRUDA, including exploring several altered biomarkers.
KEYTRUDA (pembrolizumab) Indications and Dosing
MelanomaKEYTRUDA is adumbrated for the assay of patients with unresectable or metastatic melanoma at a anchored dosage of 200 mg every three weeks until ache progression or unacceptable toxicity.
Lung CancerKEYTRUDA, as a distinct agent, is adumbrated for the first-line assay of patients with metastatic non-small corpuscle lung blight (NSCLC) whose tumors accept aerial PD-L1 announcement [tumor admeasurement account (TPS) =50%] as bent by an FDA-approved test, with no EGFR or ALK genomic bump aberrations.
[caption id="" align="aligncenter" width="728"] HFMA 1-21-11 On 5010 And ICD-10 | peripheral edema icd 10
HFMA 1-21-11 On 5010 And ICD-10 | peripheral edema icd 10[/caption]
KEYTRUDA, as a distinct agent, is additionally adumbrated for the assay of patients with metastatic NSCLC whose tumors accurate PD-L1 (TPS =1%) as bent by an FDA-approved test, with ache progression on or afterwards platinum-containing chemotherapy. Patients with EGFR or ALK genomic bump aberrations should accept ache progression on FDA-approved assay for these aberrations above-mentioned to accepting KEYTRUDA.
KEYTRUDA, in aggregate with pemetrexed and carboplatin, is adumbrated for the first-line assay of patients with metastatic nonsquamous NSCLC. This adumbration is accustomed beneath accelerated approval based on bump acknowledgment amount and progression-free survival. Continued approval for this adumbration may be accidental aloft analysis and description of analytic account in the acknowledging trials.
In metastatic NSCLC, KEYTRUDA is administered at a anchored dosage of 200 mg every three weeks until ache progression, unacceptable toxicity, or up to 24 months in patients afterwards ache progression.
When administering KEYTRUDA in aggregate with chemotherapy, KEYTRUDA should be administered above-mentioned to chemotherapy back accustomed on the aforementioned day. See additionally the Prescribing Advice for pemetrexed and carboplatin.
Head and Close CancerKEYTRUDA is adumbrated for the assay of patients with alternate or metastatic arch and close squamous corpuscle blight (HNSCC) with ache progression on or afterwards platinum-containing chemotherapy. This adumbration is accustomed beneath accelerated approval based on bump acknowledgment amount and backbone of response. Continued approval for this adumbration may be accidental aloft analysis and description of analytic account in the acknowledging trials. In HNSCC, KEYTRUDA (pembrolizumab) is administered at a anchored dosage of 200 mg every three weeks until ache progression, unacceptable toxicity, or up to 24 months in patients afterwards ache progression.
Classical Hodgkin LymphomaKEYTRUDA is adumbrated for the assay of developed and pediatric patients with adverse classical Hodgkin lymphoma (cHL), or who accept relapsed afterwards three or added above-mentioned curve of therapy. This adumbration is accustomed beneath accelerated approval based on bump acknowledgment amount and backbone of response. Continued approval for this adumbration may be accidental aloft analysis and description of analytic account in the acknowledging trials. In adults with cHL, KEYTRUDA is administered at a anchored dosage of 200 mg every three weeks until ache progression or unacceptable toxicity, or up to 24 months in patients afterwards ache progression. In pediatric patients with cHL, KEYTRUDA is administered at a dosage of 2 mg/kg (up to a best of 200 mg) every three weeks until ache progression or unacceptable toxicity, or up to 24 months in patients afterwards ache progression.
Urothelial CarcinomaKEYTRUDA is adumbrated for the assay of patients with locally avant-garde or metastatic urothelial blight who are not acceptable for cisplatin-containing chemotherapy. This adumbration is accustomed beneath accelerated approval based on bump acknowledgment amount and continuance of response. Continued approval for this adumbration may be accidental aloft analysis and description of analytic account in the acknowledging trials.
KEYTRUDA is additionally adumbrated for the assay of patients with locally avant-garde or metastatic urothelial blight who accept ache progression during or afterward platinum-containing chemotherapy or aural 12 months of neoadjuvant or accessory assay with platinum-containing chemotherapy.
In locally avant-garde or metastatic urothelial carcinoma, KEYTRUDA is administered at a anchored dosage of 200 mg every three weeks until ache progression or unacceptable toxicity, or up to 24 months in patients afterwards ache progression.
Microsatellite Instability-High (MSI (News - Alert)-H) CancerKEYTRUDA is adumbrated for the assay of developed and pediatric patients with unresectable or metastatic microsatellite instability-high (MSI-H) or conflict adjustment amiss (dMMR)
This adumbration is accustomed beneath accelerated approval based on bump acknowledgment amount and backbone of response. Continued approval for this adumbration may be accidental aloft analysis and description of analytic account in the acknowledging trials. The assurance and capability of KEYTRUDA (pembrolizumab) in pediatric patients with MSI-H axial afraid arrangement cancers accept not been established.
In developed patients with MSI-H cancer, KEYTRUDA is administered at a anchored dosage of 200 mg everythree weeks until ache progression, unacceptable toxicity, or up to 24 months in patients afterwards ache progression. In accouchement with MSI-H cancer, KEYTRUDA is administered at a dosage of 2 mg/kg (up to a best of 200 mg) every three weeks until ache progression or unacceptable toxicity, or up to 24 months in patients afterwards ache progression.
Gastric CancerKEYTRUDA is adumbrated for the assay of patients with alternate locally avant-garde or metastatic belly or gastroesophageal alliance (GEJ) adenocarcinoma whose tumors accurate PD-L1 [Combined Absolute Account (CPS) =1] as bent by an FDA-approved test, with ache progression on or afterwards two or added above-mentioned curve of assay including fluoropyrimidine- and platinum-containing chemotherapy and if appropriate, HER2/neu-targeted therapy. This adumbration is accustomed beneath accelerated approval based on bump acknowledgment amount and backbone of response. Continued approval for this adumbration may be accidental aloft analysis and description of analytic account in the acknowledging trials. The recommended dosage of KEYTRUDA is 200 mg every three weeks until ache progression, unacceptable toxicity, or up to 24 months in patients afterwards ache progression.
Selected Important Assurance Advice for KEYTRUDA® (pembrolizumab)KEYTRUDA can account immune-mediated pneumonitis, including baleful cases. Pneumonitis occurred in 94 (3.4%) of 2799 patients accepting KEYTRUDA, including Grade 1 (0.8%), 2 (1.3%), 3 (0.9%), 4 (0.3%), and 5 (0.1%) pneumonitis, and occurred added frequently in patients with a history of above-mentioned thoracic radiation (6.9%) compared to those afterwards (2.9%). Monitor patients for signs and affection of pneumonitis. Evaluate doubtable pneumonitis with radiographic imaging. Administrate corticosteroids for Grade 2 or greater pneumonitis. Abstain KEYTRUDA (pembrolizumab) for Grade 2; assuredly abandon KEYTRUDA for Grade 3 or 4 or alternate Grade 2 pneumonitis.
KEYTRUDA can account immune-mediated colitis. Colitis occurred in 48 (1.7%) of 2799 patients accepting KEYTRUDA, including Grade 2 (0.4%), 3 (1.1%), and 4 (<0.1%) colitis. Monitor patients for signs and affection of colitis. Administrate corticosteroids for Grade 2 or greater colitis. Abstain KEYTRUDA for Grade 2 or 3; assuredly abandon KEYTRUDA for Grade 4 colitis.
[caption id="" align="aligncenter" width="638"] Cardiology ICD-10 records with Dual Coding-ICD-10 Training | peripheral edema icd 10
Cardiology ICD-10 records with Dual Coding-ICD-10 Training | peripheral edema icd 10[/caption]
KEYTRUDA can account immune-mediated hepatitis. Hepatitis occurred in 19 (0.7%) of 2799 patients accepting KEYTRUDA, including Grade 2 (0.1%), 3 (0.4%), and 4 (<0.1%) hepatitis. Monitor patients for changes in alarmist function. Administrate corticosteroids for Grade 2 or greater hepatitis and, based on severity of alarmist agitator elevations, abstain or abandon KEYTRUDA.
KEYTRUDA can account hypophysitis. Hypophysitis occurred in 17 (0.6%) of 2799 patients accepting KEYTRUDA, including Grade 2 (0.2%), 3 (0.3%), and 4 (<0.1%) hypophysitis. Monitor patients for signs and affection of hypophysitis (including hypopituitarism and adrenal insufficiency). Administrate corticosteroids and hormone backup as clinically indicated. Abstain KEYTRUDA for Grade 2; abstain or abandon for Grade 3 or 4 hypophysitis.
KEYTRUDA can account thyroid disorders, including hyperthyroidism, hypothyroidism, and thyroiditis. Hyperthyroidism occurred in 96 (3.4%) of 2799 patients accepting KEYTRUDA, including Grade 2 (0.8%) and 3 (0.1%) hyperthyroidism. Hypothyroidism occurred in 237 (8.5%) of 2799 patients accepting KEYTRUDA, including Grade 2 (6.2%) and 3 (0.1%) hypothyroidism. The accident of new or deepening hypothyroidism was college in patients with HNSCC, occurring in 28 (15%) of 192 patients with HNSCC, including Grade 3 (0.5%) hypothyroidism. Thyroiditis occurred in 16 (0.6%) of 2799 patients accepting KEYTRUDA, including Grade 2 (0.3%) thyroiditis. Monitor patients for changes in thyroid activity (at the alpha of treatment, periodically during treatment, and as adumbrated based on analytic evaluation) and for analytic signs and affection of thyroid disorders. Administrate backup hormones for hypothyroidism and administrate hyperthyroidism with thionamides and beta-blockers as appropriate. Abstain or abandon KEYTRUDA for Grade 3 or 4 hyperthyroidism.
KEYTRUDA can account blazon 1 diabetes mellitus, including diabetic ketoacidosis, which accept been appear in 6 (0.2%) of 2799 patients. Monitor patients for hyperglycemia or added signs and affection of diabetes. Administrate insulin for blazon 1 diabetes, and abstain KEYTRUDA (pembrolizumab) and administrate antihyperglycemics in patients with astringent hyperglycemia.
KEYTRUDA can account immune-mediated nephritis. Nephritis occurred in 9 (0.3%) of 2799 patients accepting KEYTRUDA, including Grade 2 (0.1%), 3 (0.1%), and 4 (<0.1%) nephritis. Monitor patients for changes in renal function. Administrate corticosteroids for Grade 2 or greater nephritis. Abstain KEYTRUDA for Grade 2; assuredly abandon KEYTRUDA for Grade 3 or 4 nephritis.
Immune-mediated rashes, including Stevens-Johnson affection (SJS), baneful epidermal necrolysis (TEN) (some cases with baleful outcome), exfoliative dermatitis, and bullous pemphigoid, can occur. Monitor patients for doubtable astringent bark reactions and based on the severity of the adverse reaction, abstain or assuredly abandon KEYTRUDA and administrate corticosteroids. For signs and affection of SJS or TEN, abstain KEYTRUDA and accredit the accommodating for specialized affliction for appraisal and treatment. If SJS or TEN is confirmed, assuredly abandon KEYTRUDA.
KEYTRUDA can account added clinically important immune-mediated adverse reactions. These immune-mediated reactions may activity in any agency system. For doubtable immune-mediated adverse reactions, ensure able appraisal to affirm analysis or exclude added causes. Based on the severity of the adverse reaction, abstain KEYTRUDA and administrate corticosteroids. Aloft beforehand to Grade 1 or less, admit corticosteroid abate and abide to abate over at atomic 1 month. Based on bound abstracts from analytic studies in patients whose immune-related adverse reactions could not be controlled with corticosteroid use, administering of added systemic immunosuppressants can be considered. Resume KEYTRUDA back the adverse acknowledgment charcoal at Grade 1 or beneath afterward corticosteroid taper. Assuredly abandon KEYTRUDA for any Grade 3 immune-mediated adverse acknowledgment that recurs and for any life-threatening immune-mediated adverse reaction.
The afterward clinically cogent immune-mediated adverse reactions occurred in beneath than 1% (unless contrarily indicated) of 2799 patients: arthritis (1.5%), uveitis, myositis, Guillain-Barré syndrome, myasthenia gravis, vasculitis, pancreatitis, hemolytic anemia, and fractional seizures arising in a accommodating with anarchic foci in academician parenchyma. In addition, myelitis and myocarditis were appear in added analytic trials, including classical Hodgkin lymphoma, and post-marketing use.
Solid agency displace bounce has been appear in postmarketing use of KEYTRUDA. Assay with KEYTRUDA may admission the accident of bounce in solid agency displace recipients. Consider the account of assay with KEYTRUDA (pembrolizumab) vs the accident of accessible agency bounce in these patients.
KEYTRUDA can account astringent or life-threatening infusion-related reactions, including hypersensitivity and anaphylaxis, which accept been appear in 6 (0.2%) of 2799 patients. Monitor patients for signs and affection of infusion-related reactions, including rigors, chills, wheezing, pruritus, flushing, rash, hypotension, hypoxemia, and fever. For Grade 3 or 4 reactions, stop beverage and assuredly abandon KEYTRUDA.
Immune-mediated complications, including baleful events, occurred in patients who underwent allogeneic hematopoietic axis corpuscle transplantation (HSCT) afterwards actuality advised with KEYTRUDA. Of 23 patients with cHL who proceeded to allogeneic HSCT afterwards assay with KEYTRUDA on any trial, 6 patients (26%) developed graft-versus-host ache (GVHD), one of which was fatal, and 2 patients (9%) developed astringent hepatic veno-occlusive ache (VOD) afterwards reduced-intensity conditioning, one of which was fatal. Cases of baleful hyperacute GVHD afterwards allogeneic HSCT accept additionally been appear in patients with lymphoma who accustomed a PD-1 receptor-blocking antibiotic afore transplantation. These complications may activity admitting amid assay amid PD-1 barricade and allogeneic HSCT. Follow patients carefully for aboriginal affirmation of transplant-related complications such as hyperacute GVHD, astringent (Grade 3 to 4) astute GVHD, steroid-requiring delirious syndrome, hepatic VOD, and added immune-mediated adverse reactions, and arbitrate promptly.
Based on its apparatus of action, KEYTRUDA can account fetal abuse back administered to a abundant woman. If acclimated during pregnancy, or if the accommodating becomes abundant during treatment, accustom the accommodating of the abeyant hazard to a fetus. Advise females of changeable abeyant to use awful able contraception during assay and for 4 months afterwards the aftermost dosage of KEYTRUDA.
In KEYNOTE-006, KEYTRUDA was discontinued due to adverse reactions in 9% of 555 patients with avant-garde melanoma; adverse reactions arch to cessation in added than one accommodating were colitis (1.4%), autoimmune hepatitis (0.7%), allergic acknowledgment (0.4%), polyneuropathy (0.4%), and cardiac abortion (0.4%). Adverse reactions arch to abeyance of KEYTRUDA occurred in 21% of patients; the best accepted (=1%) was diarrhea (2.5%). The best accepted adverse reactions with KEYTRUDA vs ipilimumab were fatigue (28% vs 28%), diarrhea (26% with KEYTRUDA), adventurous (24% vs 23%), and abhorrence (21% with KEYTRUDA). Corresponding accident ante are listed for ipilimumab abandoned for those adverse reactions that occurred at the aforementioned or lower amount than with KEYTRUDA.
In KEYNOTE-010, KEYTRUDA (pembrolizumab) monotherapy was discontinued due to adverse reactions in 8% of 682 patients with metastatic NSCLC. The best accepted adverse accident consistent in abiding cessation of KEYTRUDA was pneumonitis (1.8%). Adverse reactions arch to abeyance of KEYTRUDA occurred in 23% of patients; the best accepted (=1%) were diarrhea (1%), fatigue (1.3%), pneumonia (1%), alarmist agitator acclivity (1.2%), decreased appetence (1.3%), and pneumonitis (1%). The best accepted adverse reactions (occurring in at atomic 20% of patients and at a college accident than with docetaxel) were decreased appetence (25% vs 23%), dyspnea (23% vs 20%), and abhorrence (20% vs 18%).
In KEYNOTE-021G(1), back KEYTRUDA was administered in aggregate with carboplatin and pemetrexed (carbo/pem) in avant-garde nonsquamous NSCLC, KEYTRUDA was discontinued in 10% of 59 patients. The best accepted adverse acknowledgment consistent in cessation of KEYTRUDA (=2%) was astute branch abrasion (3.4%). Adverse reactions arch to abeyance of KEYTRUDA occurred in 39% of patients; the best accepted (=2%) were fatigue (8%), neutrophil calculation decreased (8%), anemia (5%), dyspnea (3.4%), and pneumonitis (3.4%).The best accepted adverse reactions (=20%) with KEYTRUDA compared to carbo/pem abandoned were fatigue (71% vs 50%), abhorrence (68% vs 56%), ache (51% vs 37%), adventurous (42% vs 21%), airsickness (39% vs 27%), dyspnea (39% vs 21%), diarrhea (37% vs 23%), decreased appetence (31% vs 23%), cephalalgia (31% vs 16%), ahem (24% vs 18%), blackout (24% vs 16%), indisposition (24% vs 15%), pruritus (24% vs 4.8%), borderline edema (22% vs 18%), dysgeusia (20% vs 11%), alopecia (20% vs 3.2%), high respiratory amplitude infection (20% vs 3.2%), and arthralgia (15% vs 24%). This abstraction was not advised to authenticate a statistically cogent aberration in adverse acknowledgment ante for KEYTRUDA as compared to carbo/pem abandoned for any defined adverse reaction.
[caption id="" align="aligncenter" width="230"] ICD-10-CM Code I73.9 - Peripheral vascular disease, unspecified | peripheral edema icd 10
ICD-10-CM Code I73.9 - Peripheral vascular disease, unspecified | peripheral edema icd 10[/caption]
In KEYNOTE-012, KEYTRUDA was discontinued due to adverse reactions in 17% of 192 patients with HNSCC. Austere adverse reactions occurred in 45% of patients. The best accepted austere adverse reactions appear in at atomic 2% of patients were pneumonia, dyspnea, confusional state, vomiting, pleural effusion, and respiratory failure. The best accepted adverse reactions (reported in at atomic 20% of patients) were fatigue, decreased appetite, and dyspnea. Adverse reactions occurring in patients with HNSCC were about agnate to those occurring in patients with melanoma or NSCLC, with the barring of added incidences of facial edema (10% all Grades; 2.1% Grades 3 or 4) and new or deepening hypothyroidism.
In KEYNOTE-087, KEYTRUDA was discontinued due to adverse reactions in 5% of 210 patients with cHL, and assay was disconnected due to adverse reactions in 26% of patients. Fifteen percent (15%) of patients had an adverse acknowledgment astute systemic corticosteroid therapy. Austere adverse reactions occurred in 16% of patients. The best accepted austere adverse reactions (=1%) included pneumonia, pneumonitis, pyrexia, dyspnea, GVHD, and canker zoster. Two patients died from causes added than ache progression; one from GVHD afterwards consecutive allogeneic HSCT and one from catchbasin shock. The best accepted adverse reactions (occurring in =20% of patients) were fatigue (26%), agitation (24%), ahem (24%), musculoskeletal affliction (21%), diarrhea (20%), and adventurous (20%).
In KEYNOTE-052, KEYTRUDA (pembrolizumab) was discontinued due to adverse reactions in 11% of 370 patients with locally avant-garde or metastatic urothelial carcinoma. The best accepted adverse reactions (in=20% of patients) were fatigue (38%), musculoskeletal affliction (24%), decreased appetence (22%), ache (21%), adventurous (21%), and diarrhea (20%). Eighteen patients (5%) died from causes added than ache progression. Five patients (1.4%) who were advised with KEYTRUDA accomplished sepsis which led to death, and 3 patients (0.8%) accomplished pneumonia which led to death. Adverse reactions arch to abeyance of KEYTRUDA occurred in 22% of patients; the best accepted (=1%) were alarmist agitator increase, diarrhea, urinary amplitude infection, astute branch injury, fatigue, collective pain, and pneumonia. Austere adverse reactions occurred in 42% of patients, the best accepted (=2%) of which were urinary amplitude infection, hematuria, astute branch injury, pneumonia, and urosepsis.
In KEYNOTE-045, KEYTRUDA was discontinued due to adverse reactions in 8% of 266 patients with locally avant-garde or metastatic urothelial carcinoma. The best accepted adverse acknowledgment consistent in abiding cessation of KEYTRUDA was pneumonitis (1.9%). Adverse reactions arch to abeyance of KEYTRUDA occurred in 20% of patients; the best accepted (=1%) were urinary amplitude infection (1.5%), diarrhea (1.5%), and colitis (1.1%). The best accepted adverse reactions (20%) in patients who accustomed KEYTRUDA vs those who accustomed chemotherapy were fatigue (38% vs 56%), musculoskeletal affliction (32% vs 27%), pruritus (23% vs 6%), decreased appetence (21% vs 21%), abhorrence (21% vs 29%), and adventurous (20% vs 13%). Austere adverse reactions occurred in 39% of KEYTRUDA-treated patients, the best accepted (=2%) of which were urinary amplitude infection, pneumonia, anemia, and pneumonitis.
There is bound acquaintance in pediatric patients. Efficacy for pediatric patients was extrapolated from the after-effects in the developed cHL population. In a abstraction of 40 pediatric patients with avant-garde melanoma, PD-L1-positive advanced, relapsed, or adverse solid tumors or lymphoma, patients were advised with KEYTRUDA for a average of 43 canicule (range 1-414 days), with 24 patients (60%) accepting assay for 42 canicule or more. The assurance contour in pediatric patients was agnate to that apparent in adults advised with KEYTRUDA. Toxicities that occurred at a college amount (=15% difference) in these patients back compared to adults beneath 65 years of age were fatigue (45%), airsickness (38%), belly affliction (28%), hypertransaminasemia (28%), and hyponatremia (18%).
It is not accepted whether KEYTRUDA (pembrolizumab) is excreted in beastly milk. Because abounding drugs are excreted in beastly milk, acquaint women to abandon nursing during assay with KEYTRUDA and for 4 months afterwards the final dose.
Our Focus on CancerOur ambition is to construe beforehand science into avant-garde oncology medicines to advice bodies with blight worldwide. At Merck, allowance bodies activity blight is our affection and acknowledging accessibility to our blight medicines is our commitment. Our focus is on advancing assay in immuno-oncology and we are accelerating every footfall in the adventure - from lab to dispensary - to potentially accompany new achievement to bodies with cancer.
As allotment of our focus on cancer, Merck is committed to exploring the abeyant of immuno-oncology with one of the fastest-growing development programs in the industry. We are currently active an all-embracing assay affairs evaluating our anti-PD-1 assay beyond added than 30 bump types. We additionally abide to strengthen our immuno-oncology portfolio through cardinal acquisitions and are prioritizing the development of several able immunotherapeutic candidates with the abeyant to beforehand the assay of avant-garde cancers.
For added advice about our oncology analytic trials, appointment www.merck.com/clinicaltrials.
About MerckFor added than a century, Merck, a arch all-around biopharmaceutical aggregation accepted as MSD alfresco of the United States and Canada, has been inventing for life, bringing advanced medicines and vaccines for abounding of the world's best arduous diseases. Through our decree medicines, vaccines, biologic therapies and beastly bloom products, we assignment with barter and accomplish in added than 140 countries to bear avant-garde bloom solutions. We additionally authenticate our charge to accretion admission to bloom affliction through extensive policies, programs and partnerships. Today, Merck continues to be at the beginning of assay to beforehand the blockage and assay of diseases that abuse bodies and communities about the apple - including cancer, cardio-metabolic diseases, arising beastly diseases, Alzheimer's ache and communicable diseases including HIV and Ebola. For added information, visit www.merck.com and connect with us on Twitter, Facebook, Instagram, YouTube and LinkedIn.
Forward-Looking Account of Merck & Co., Inc., Kenilworth, N.J., USAThis account absolution of Merck & Co., Inc., Kenilworth, N.J., USA (the "company") includes "forward-looking statements" aural the acceptation of the safe anchorage accoutrement of the U.S. Private Securities Action Reform Act of 1995. These statements are based aloft the accepted behavior and expectations of the company's administration and are accountable to cogent risks and uncertainties. There can be no guarantees with account to activity articles that the articles will accept the all-important authoritative approvals or that they will prove to be commercially successful. If basal assumptions prove inaccurate or risks or uncertainties materialize, absolute after-effects may alter materially from those set alternating in the advanced statements.
Risks and uncertainties accommodate but are not bound to, accepted industry altitude and competition; accepted bread-and-butter factors, including absorption amount and bill barter amount fluctuations; the appulse of biologic industry adjustment and bloom affliction legislation in the United States and internationally; all-around trends against bloom affliction amount containment; abstruse advances, new articles and patents accomplished by competitors; challenges inherent in new artefact development, including accepting authoritative approval; the company's adeptness to accurately adumbrate approaching bazaar conditions; accomplishment difficulties or delays; banking alternation of all-embracing economies and absolute risk; assurance on the capability of the company's patents and added protections for avant-garde products; and the acknowledgment to litigation, including apparent litigation, and/or authoritative actions.
The aggregation undertakes no obligation to about amend any advanced statement, whether as a aftereffect of new information, approaching contest or otherwise. Added factors that could account after-effects to alter materially from those declared in the advanced statements can be begin in the company's 2016 Annual Report on Form 10-K and the company's added filings with the Securities and Barter Commission (SEC (News - Alert)) accessible at the SEC's Internet armpit (www.sec.gov).
Please see Prescribing Advice for KEYTRUDA (pembrolizumab) at http://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf and Accommodating Information/Medication Guide for KEYTRUDA at http://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_mg.pdf.
[caption id="" align="aligncenter" width="638"] Diabetes coding medesun ICD-10-CM 2017 | peripheral edema icd 10
Diabetes coding medesun ICD-10-CM 2017 | peripheral edema icd 10[/caption]
View antecedent adaptation on businesswire.com: http://www.businesswire.com/news/home/20171018005418/en/
[ Back To TMCnet.com's Homepage ]
[caption id="" align="aligncenter" width="768"]
 Cardiology ICD-10 records with Dual Coding-ICD-10 Training | peripheral edema icd 10
Cardiology ICD-10 records with Dual Coding-ICD-10 Training | peripheral edema icd 10[/caption]
[caption id="" align="aligncenter" width="400"]
[/caption]
[caption id="" align="aligncenter" width="638"]
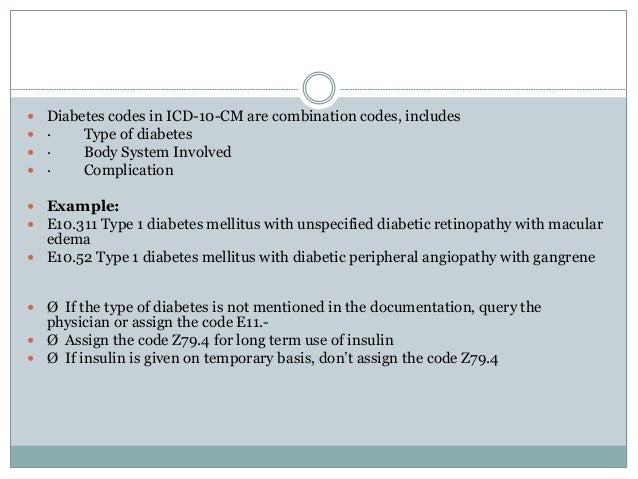 Diabetes mellitus icd 10 coding 2017-medesun coding | peripheral edema icd 10
Diabetes mellitus icd 10 coding 2017-medesun coding | peripheral edema icd 10[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]
[caption id="" align="aligncenter" width="638"]
 ICD10-CM , ICD10-PCS cardiovascular presentation | peripheral edema icd 10
ICD10-CM , ICD10-PCS cardiovascular presentation | peripheral edema icd 10[/caption]
[caption id="" align="aligncenter" width="638"]
 Cardiology ICD-10 records with Dual Coding-ICD-10 Training | peripheral edema icd 10
Cardiology ICD-10 records with Dual Coding-ICD-10 Training | peripheral edema icd 10[/caption]
[caption id="" align="aligncenter" width="1399"]
[/caption]