These data demonstrate that the known and potential benefits of this vaccine outweigh the known and potential harms of becoming infected with the coronavirus disease 2019 COVID 19. Most in the study were younger than 55 but the results so far.
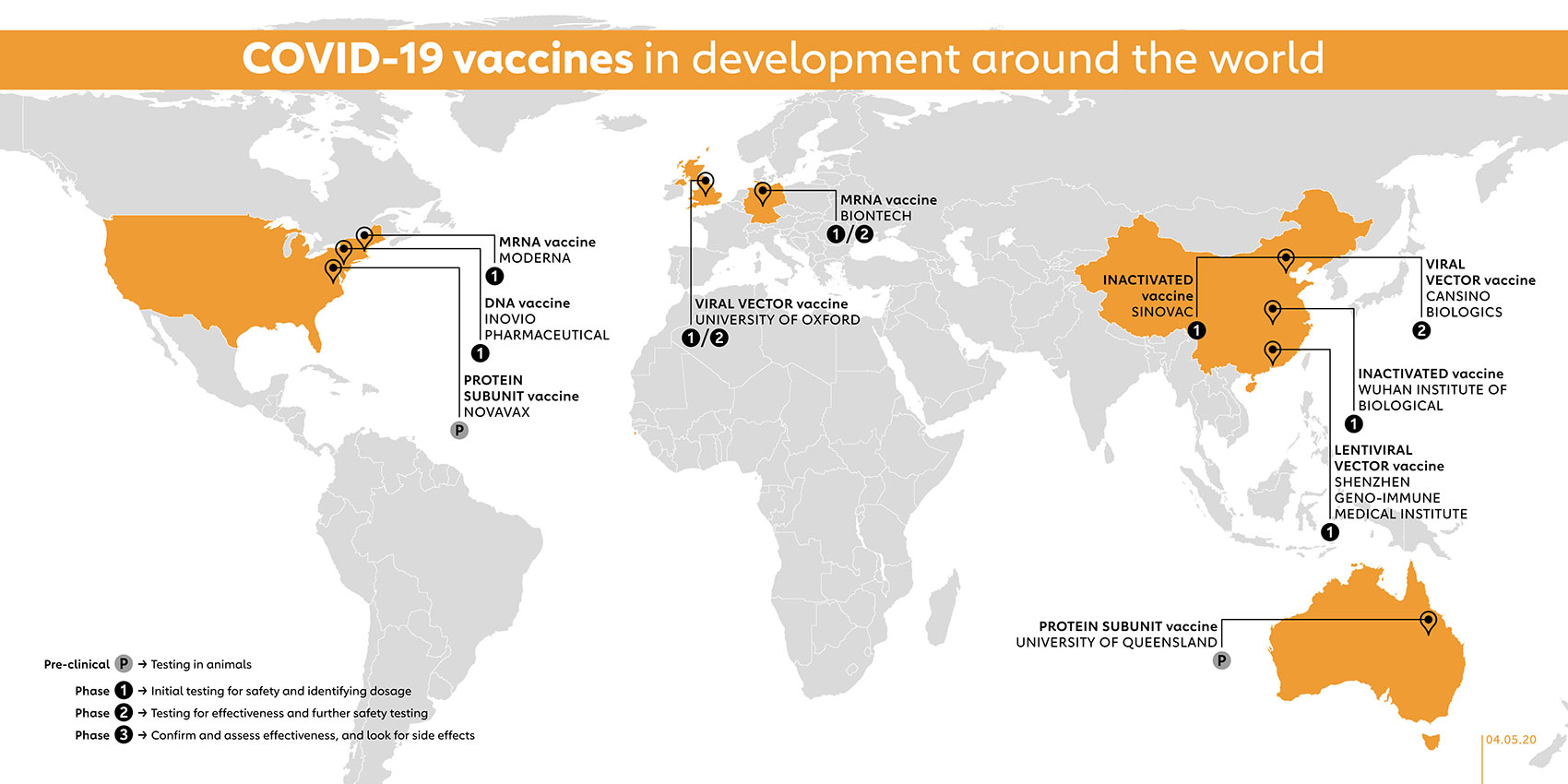 1 2 Update On Treatments And Vaccines Under Development
1 2 Update On Treatments And Vaccines Under Development
The primary end points were efficacy of the vaccine against laboratory-confirmed Covid-19 and safety.
Covid vaccine efficacy and safety. Some people are asking if the COVID-19 vaccines are safe. Thus the RR for Moderna vaccine up to this. Even though care home residents and older people are likely to be amongst the first to be vaccinated these patient groups are usually excluded from clinical trials.
Safety of COVID-19 Vaccines. The COVID-19 mRNA vaccines are being held to the same. BNT162b2 is a lipid nanoparticle-formulated nucleoside-modified RNA vaccine that encodes a prefusion stabilized membrane-anchored SARS-CoV-2 full-length spike protein.
The study published in the New England Journal of Medicine 1 found that vaccine efficacy between the first and second doses was 52 95 credible interval 295 to 684 with 39 cases of covid-19 in the vaccine group and 82 cases in the placebo group. Funded by the Biomedical Advanced Research and Development Authority and the National Institute of Allergy and. Investigators of four randomised controlled trials conducted in the UK South Africa and Brazil report pooled results of an interim analysis of safety and efficacy against COVID-19 of the OxfordAstraZeneca chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19 AZD1222 in adults aged 18 years and older.
The COVID-19 vaccine for instance cannot give someone the virus and cannot affect or interact with our DNA or change our genes in any way. New vaccine efficacy results are reported now in The Lancet. A heterologous recombinant adenovirus rAd-based vaccine Gam-COVID-Vac Sputnik V showed a good safety profile and induced strong humoral and cellular immune responses in participants in phase 12 clinical trials.
The OxfordAstraZeneca Covid vaccine is safe and effective giving good protection researchers have confirmed in The Lancet journal. An effective COVID-19 vaccine will help protect people who come in contact with the virus from becoming sick. Their safety and efficacy in older people is critical to their success.
Of the first 95 to develop Covid-19 symptoms in the Moderna trial only five were in the vaccine group and the remaining 90 were in the placebo group. The mRNA-1273 vaccine showed 941 efficacy at preventing Covid-19 illness including severe disease. Food and Drug Administration FDA has granted Emergency Use Authorizations EUA for two COVID-19 vaccines which have been shown to be safe and effective as determined by data from the manufacturers and findings from large clinical trials.
The Coronavirus Efficacy COVE phase 3 trial was launched in late July 2020 to assess the safety and efficacy of the mRNA-1273 vaccine in preventing SARS-CoV-2 infection. An independent data and. Nevertheless in the interval between the first and second doses the observed vaccine efficacy against Covid-19 was 52 and in the first 7 days after dose 2 it was 91 reaching full efficacy.
Other open questions about the rapidly developed COVID-19 vaccines include long-term safety indicating the critical need for pharmacovigilance activities the duration of vaccine protection the. As more people are vaccinated families and communities will be able to gradually return to a more normal routine. Aside from transient local and systemic reactions no safety concerns were identified.
Here we report preliminary results on the efficacy and safety of Gam-COVID-Vac from the interim analysis of this phase 3 trial. Several vaccines against coronavirus disease 2019 COVID-19 are on the cusp of regulatory approval.
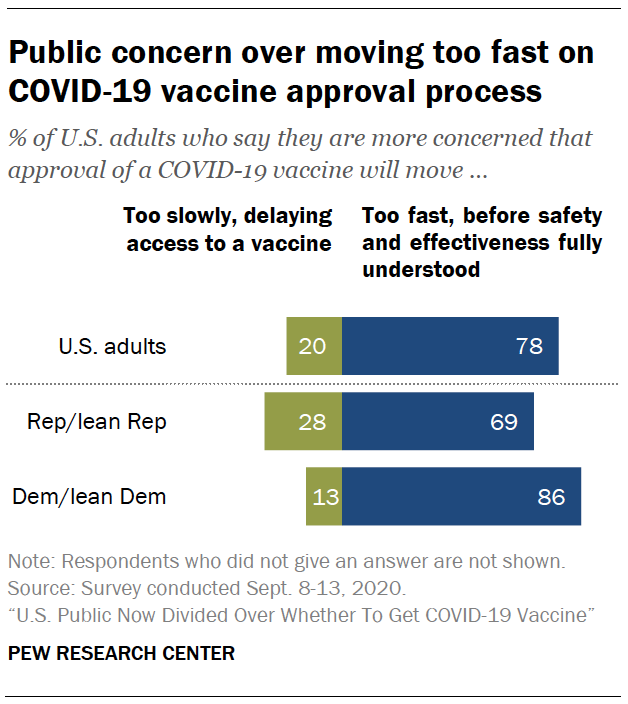 U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
 Pfizer Covid Vaccine Is 95 Effective Plans To Submit To Fda In Days
Pfizer Covid Vaccine Is 95 Effective Plans To Submit To Fda In Days
Monitoring Vaccine Uptake Safety And Effectiveness
 Initial Vaccine Dose Offers Some Protection But Not Enough For 95 Efficacy
Initial Vaccine Dose Offers Some Protection But Not Enough For 95 Efficacy
 A Strategic Approach To Covid 19 Vaccine R D Science
A Strategic Approach To Covid 19 Vaccine R D Science

 Russian Medics Will Get Coronavirus Vaccine As Fauci Doubts Its Safety And Effectiveness
Russian Medics Will Get Coronavirus Vaccine As Fauci Doubts Its Safety And Effectiveness
 China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
 Coronavirus Peru And Morocco To Start Phase 3 Trials Of Two Chinese Vaccine Hopefuls South China Morning Post
Coronavirus Peru And Morocco To Start Phase 3 Trials Of Two Chinese Vaccine Hopefuls South China Morning Post
 Worried About The Safety And Effectiveness Of The Covid 19 Vaccine The Save Act Aims To Make Transparency A Priority
Worried About The Safety And Effectiveness Of The Covid 19 Vaccine The Save Act Aims To Make Transparency A Priority
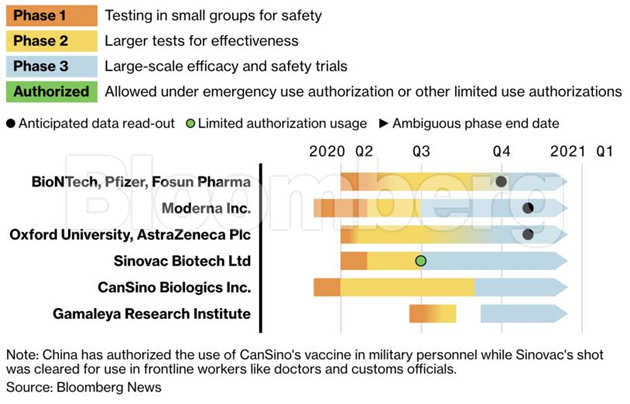
 Covid Vaccine Tracker American Lung Association
Covid Vaccine Tracker American Lung Association
 Experts Weigh In On Uk S Untested Coronavirus Vaccination Strategy Business Insider
Experts Weigh In On Uk S Untested Coronavirus Vaccination Strategy Business Insider
 Covid 19 Vaccine Update With Professor Michael Mina Md Safety Efficacy Distribution Trials Youtube
Covid 19 Vaccine Update With Professor Michael Mina Md Safety Efficacy Distribution Trials Youtube
 Respiratory Digests Ers European Respiratory Society
Respiratory Digests Ers European Respiratory Society
 Covid 19 Vaccine Safety Lessons Learned From Influenza National Foundation For Infectious Diseases
Covid 19 Vaccine Safety Lessons Learned From Influenza National Foundation For Infectious Diseases
 Across The Vaccine Efficacy Line And A Late Breaking Safety Episode Covid 19 Vaccine Race Month 11 Absolutely Maybe
Across The Vaccine Efficacy Line And A Late Breaking Safety Episode Covid 19 Vaccine Race Month 11 Absolutely Maybe
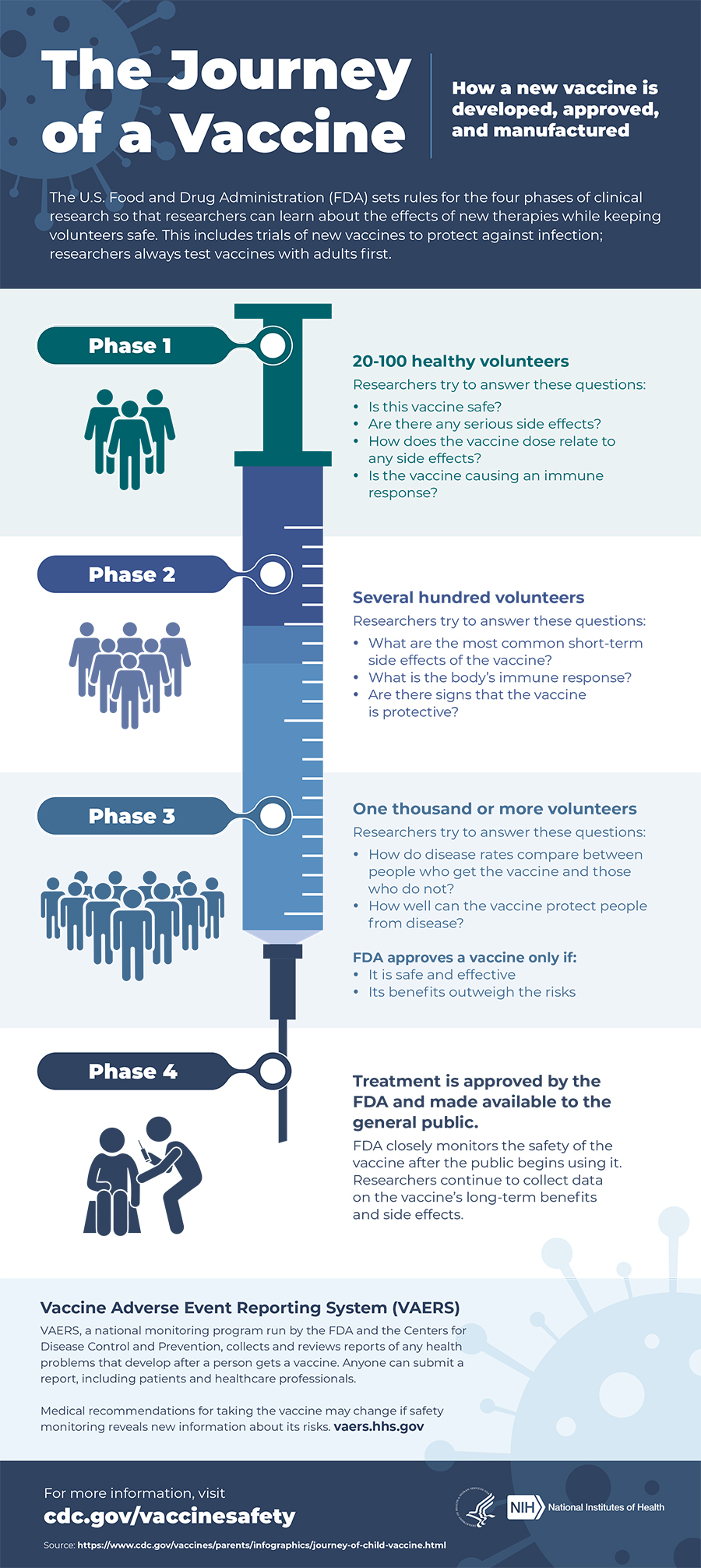 Is The Covid 19 Vaccine Safe Johns Hopkins Medicine
Is The Covid 19 Vaccine Safe Johns Hopkins Medicine
 Covid 19 Vaccine Covaxin Is Safe Developed On Time Proven Tech Says Bharat Biotech Md
Covid 19 Vaccine Covaxin Is Safe Developed On Time Proven Tech Says Bharat Biotech Md
 Covid 19 Vaccination Faq Mayo Clinic Health System
Covid 19 Vaccination Faq Mayo Clinic Health System
 Covid 19 Vaccines Verifying Safety And Identifying Misinformation Covid 19 Johns Hopkins Bloomberg School Of Public Health
Covid 19 Vaccines Verifying Safety And Identifying Misinformation Covid 19 Johns Hopkins Bloomberg School Of Public Health
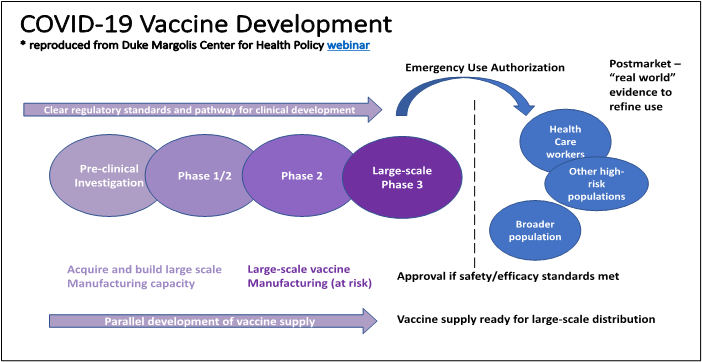 Update On Covid 19 Vaccine Development National Governors Association
Update On Covid 19 Vaccine Development National Governors Association
 Covid 19 Vaccine Explained What It Means For You Which News
Covid 19 Vaccine Explained What It Means For You Which News
 Efficacy And Safety Of The Mrna 1273 Sars Cov 2 Vaccine Nejm
Efficacy And Safety Of The Mrna 1273 Sars Cov 2 Vaccine Nejm

