Safety and efficacy of the ChAdOx1 nCoV-19 vaccine Azd1222 against SARS-CoV-2. Oxford vaccine safe and effective study shows.

The Oxford Vaccine Centres COVID-19 vaccine trial is being run by the Jenner Institute and Oxford Vaccine Group.

Covid vaccine oxford when. Published 23 November 2020. India has given the green light to the Covid-19 vaccine developed by Oxford University-AstraZeneca and three other vaccine candidates are a. The COVID-19 vaccine developed in Britain by Oxford University and AstraZeneca has been approved by the UK.
Phase 3 interim analysis including 131 Covid-19 cases indicates that the vaccine is 704 effective when combining data from two dosing regimens. The OxfordAstraZeneca COVID-19 vaccine codenamed AZD1222 is a COVID-19 vaccine developed by Oxford University and AstraZeneca given by intramuscular injection using as a vector the modified chimpanzee adenovirus ChAdOx1. A vaccine candidate for COVID-19 has been identified by researchers from the Oxford Vaccine Group and Oxfords Jenner Institute.
The team who started work on developing a vaccine to prevent COVID-19 on 20th January 2020 is led by Prof. Updated July 19 2020. The Oxford team has said a gap of up to 12 weeks between doses will provide the maximum immunity expected from its vaccine which trials showed to be over 70 effective at preventing COVID-19.
COVID-19 is a disease caused by the severe acute respiratory syndrome coronavirus 2 SARS-CoV-2. Britain today approved the OxfordAstraZeneca Covid-19 vaccine paving the way for millions to receive the jab within weeks and offering hope that the UK could be out of the coronavirus crisis. Authorizes Covid-19 Vaccine From Oxford and AstraZeneca Health officials hope to soon vaccinate up to two million people per week as the countrys hospitals are overwhelmed by cases of a.
Our vaccine work is progressing quickly. Voysey M Clemens SAC Madhi SA et al. One dosing regimen showed 90 efficacy when a half-dose was followed by a full-dose after at least one month based on mixed trials with no participants over 55 years old.
An interim analysis of four randomised controlled trials in Brazil South Africa and the UK. The main focus of the Phase I II and III studies is to assess whether the ChAdOx1 vaccine is going to work against COVID-19 that it doesnt cause unacceptable side effects and if it induces good immune responses. Our vaccine work is progressing quickly.
Trial shows Oxford Covid vaccine highly effective. Government for emergency use the pharmaceutical company announced early Wednesday morning. The OxfordAstraZeneca vaccine is based on a weakened version of a common cold virus adenovirus in chimpanzees which has been genetically changed to stop Covid-19 replicating in.
Catherine Green and Prof. About the Oxford COVID-19 vaccine. The coronavirus vaccine developed by the University of Oxford is highly effective at stopping people developing Covid-19 symptoms a large trial shows.
Updated July 19 2020. The phase I trial in healthy adult volunteers began in April. Published 8 December 2020.
To ensure you have the latest information or to find out more about the trial please visit the Oxford COVID-19 vaccine web hub or visit the COVID-19 trial website. To ensure you have the latest information or to find out more about the trial please visit the Oxford COVID-19 vaccine web hub or visit the COVID-19 trial website. More than 1000 immunisations have been completed and follow-up is currently ongoing.
Teresa Lambe Dr Sandy Douglas Prof. The Oxford COVID-19 vaccine trials.
 Covid 19 In Spain The Differences Between The Pfizer Moderna And Oxford Coronavirus Vaccines What We Know So Far Science Tech El Pais In English
Covid 19 In Spain The Differences Between The Pfizer Moderna And Oxford Coronavirus Vaccines What We Know So Far Science Tech El Pais In English
 Pfizer Biontech Covid 19 Vaccine Wikipedia
Pfizer Biontech Covid 19 Vaccine Wikipedia
 Different Age Groups May Get Different Covid Vaccines Experts Say Science The Guardian
Different Age Groups May Get Different Covid Vaccines Experts Say Science The Guardian
 Covishield Oxford S Covid Vaccine Gets Expert Panel S Nod For Emergency Use Dcgi To Take Final Call Coronavirus Outbreak News
Covishield Oxford S Covid Vaccine Gets Expert Panel S Nod For Emergency Use Dcgi To Take Final Call Coronavirus Outbreak News
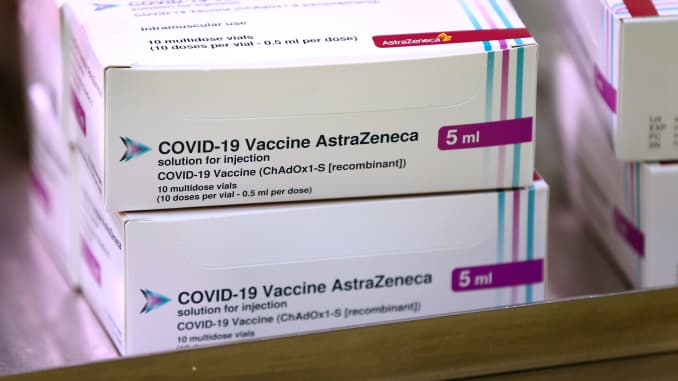 Covid Vaccine Astrazeneca S Could Be Distributed In Eu By Mid February
Covid Vaccine Astrazeneca S Could Be Distributed In Eu By Mid February
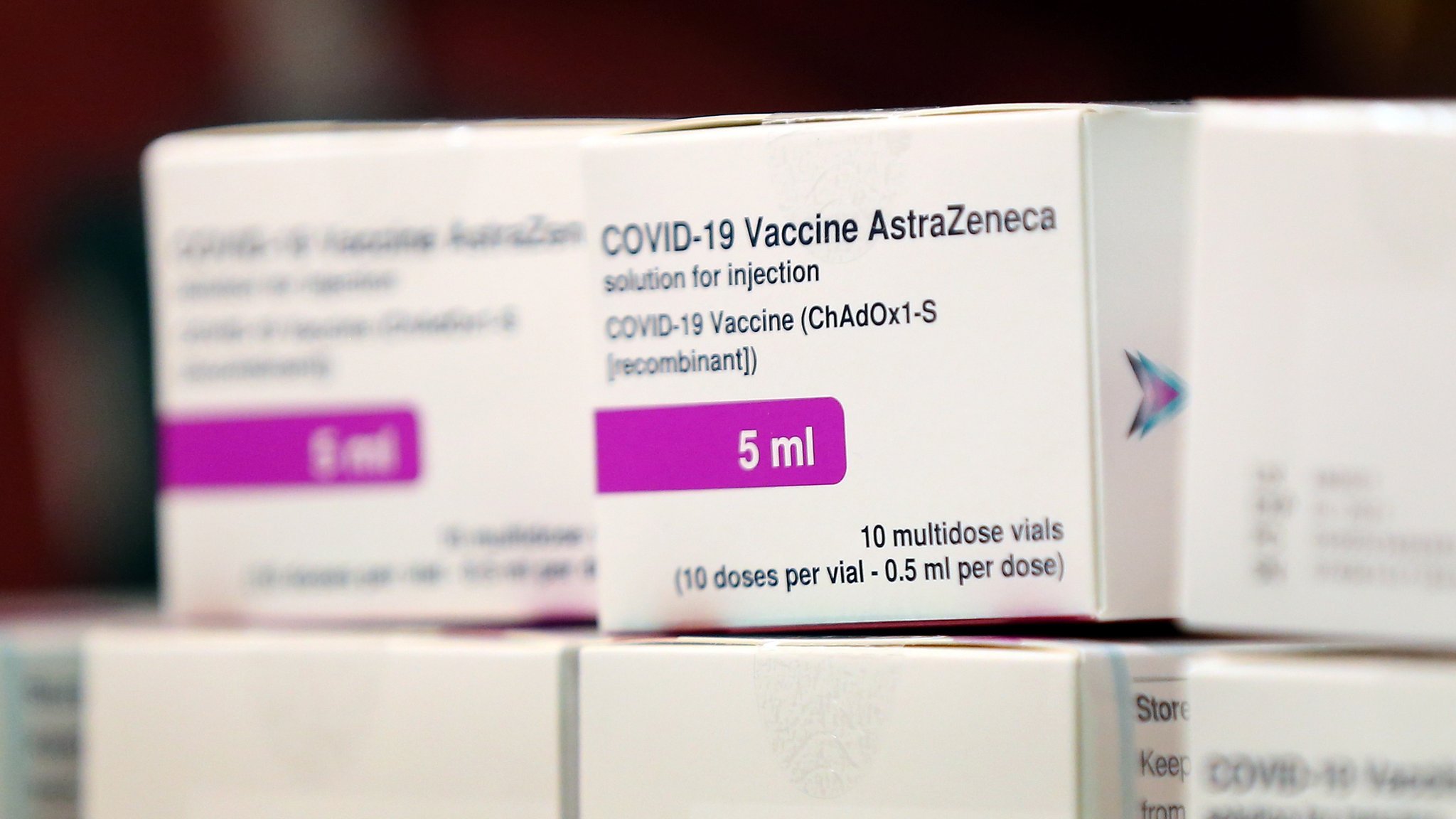 Coronavirus Vaccine Pm Expects Tens Of Millions Of Jabs By April Bbc News
Coronavirus Vaccine Pm Expects Tens Of Millions Of Jabs By April Bbc News
 India Begins Mass Immunisation Drive As Covid Vaccines Approved
India Begins Mass Immunisation Drive As Covid Vaccines Approved
 Here S Everything You Need To Know About The Potential Oxford University Covid Vaccine Australia News The Guardian
Here S Everything You Need To Know About The Potential Oxford University Covid Vaccine Australia News The Guardian
 The Uk Is Delaying Second Pfizer Biontech Shot Here S What We Know
The Uk Is Delaying Second Pfizer Biontech Shot Here S What We Know
 Coronavirus Vaccine When Will We Have One Coronavirus The Guardian
Coronavirus Vaccine When Will We Have One Coronavirus The Guardian
 Covid 19 Vaccines Vaccine Knowledge
Covid 19 Vaccines Vaccine Knowledge
 India S Covid 19 Vaccine When Will Mass Production Start And Who Will Get It First
India S Covid 19 Vaccine When Will Mass Production Start And Who Will Get It First
 The World S Biggest Coronavirus Vaccine Study Begins A U S Trial That Will Include 30 000 People To See If The Shots Really Work
The World S Biggest Coronavirus Vaccine Study Begins A U S Trial That Will Include 30 000 People To See If The Shots Really Work
 Where Is The Oxford Astrazeneca Covid Vaccine Made
Where Is The Oxford Astrazeneca Covid Vaccine Made
2 Billion Doses Of Oxford Covid 19 Vaccine Planned After Gates Funding
 Oxford Vaccine Trial On Hold Because Of Potential Safety Issue
Oxford Vaccine Trial On Hold Because Of Potential Safety Issue
 Eu Hit By Delay To Oxford Astrazeneca Vaccine Delivery Financial Times
Eu Hit By Delay To Oxford Astrazeneca Vaccine Delivery Financial Times
 Phase 3 Results In Sight For Some While One Vaccine Is Put On Hold Covid 19 Vaccine Race Month 9 Absolutely Maybe
Phase 3 Results In Sight For Some While One Vaccine Is Put On Hold Covid 19 Vaccine Race Month 9 Absolutely Maybe
Why Oxford Covid 19 Vaccine Is Cheaper Than Pfizer Moderna Jabs
 Covid 19 Vaccines What They Mean For People With Ms Ms Trust
Covid 19 Vaccines What They Mean For People With Ms Ms Trust
 Uk Approves Pfizer Biontech Covid Vaccine For Rollout Next Week Society The Guardian
Uk Approves Pfizer Biontech Covid Vaccine For Rollout Next Week Society The Guardian
 Hospitals In England Told To Prepare For Covid Vaccine Rollout In 10 Days Time Society The Guardian
Hospitals In England Told To Prepare For Covid Vaccine Rollout In 10 Days Time Society The Guardian
 Oxford Covid Vaccine Works In All Ages Trials Suggest World News The Guardian
Oxford Covid Vaccine Works In All Ages Trials Suggest World News The Guardian
