Covid-19 vaccine efficacy results are not enough What the latest Covid-19 vaccine announcements from AstraZeneca-Oxford Pfizer-BioNTech and Moderna can and cant tell us. The trial findingsconducted at a time of significant transmission in the country and an emerging more transmissible variant spreading globallycoincide with phase 2b findings which show lesser.
 Distributing Our Covid 19 Vaccine To The World Pfizer
Distributing Our Covid 19 Vaccine To The World Pfizer
M oderna said Monday that its Covid-19 vaccine continued to deliver strong efficacy results showing 94 efficacy in the main analysis of its key study.

Covid vaccine efficacy results. This first interim analysis was based on 95 cases of which 90 cases of COVID-19 were observed in the placebo group versus 5 cases observed in the mRNA-1273 group resulting in a point estimate of vaccine efficacy of 945 p. The vaccine candidate was found to be more than 90 effective in preventing COVID-19 in participants without evidence of prior SARS-CoV-2 infection in the first interim efficacy analysis. Newly published results show it is safe but questions remain over its efficacy December 9 2020 1152am EST Paul Hunter University of East Anglia.
The interim analysis comprised 95 cases of symptomatic COVID-19 among volunteers. In Pfizers large clinical trial the two doses of the vaccine were shown to be 95 effective at preventing COVID-19 cases according to results published in The New England Journal of Medicine. The results demonstrate that our mRNA-based vaccine can help prevent COVID-19 in the majority of people who receive it.
NVX-CoV2373 is the first vaccine to demonstrate not only high clinical efficacy against COVID-19 but also significant clinical efficacy against both the rapidly emerging UK and South Africa. The results demonstrate that our mRNA-based vaccine can help prevent COVID-19 in the majority of people who receive it. This interim review of the data suggests that the vaccine is safe and effective at preventing symptomatic COVID-19 in adults.
Protein-based coronavirus 2019 COVID-19 vaccine candidate NVX-CoV2373 from Novavax has reported phase 3 findings showing 893 efficacy in prevention of COVID-19 in participants from the UK. Final results from the trials of Modernas vaccine against Covid-19 confirm it has 94 efficacy and nobody who was vaccinated with it developed severe disease said the company kickstarting the. Johnson Johnsons JJ JNJ-78436735 COVID-19 vaccine was shown to be 90 efficacious in interim results according to findings published in the New England Journal of MedicineThe single dose investigational vaccine which is being developed by the Janssen Pharmaceutical Companies of Johnson Johnson is more commonly referred to as the Ad26COV2S vaccine and is a recombinant.
The disparity in the latest results means there will be considerable uncertainty over precisely how well the Oxford vaccine protects against COVID-19 until ongoing efficacy trials report more data. The company said it would immediately seek. The first full peer-reviewed results of phase 3 trials of the COVID-19 vaccine developed by AstraZeneca and Oxford University show that it is safe and up to 90 effective in preventing infection supporting regulatory submissions for emergency use.
MRK which was a late entrant to the COVID-19 vaccine fray has dropped out of the race and has instead opted to channelize resources on the development of therapeutics. The DSMB reported that the candidate was safe and well-tolerated and noted a vaccine efficacy rate of 945. By Umair Irfan Nov 24.
Merck Co Inc. The vaccine candidate was found to be more than 90 effective in preventing COVID-19 in participants without evidence of prior SARS-CoV-2 infection in the first interim efficacy analysis. The interim analysis published today in The Lancet identified no severe coronavirus disease or hospitalizations in pooled results from the.
Russia Repeats 91 4 Efficacy Rate In New Covid 19 Vaccine Data Developers Reuters

 Expert Reaction Moderna Vaccine Reports 100 Efficacy Against Severe Covid Scimex
Expert Reaction Moderna Vaccine Reports 100 Efficacy Against Severe Covid Scimex
 Efficacy And Safety Of The Mrna 1273 Sars Cov 2 Vaccine Nejm
Efficacy And Safety Of The Mrna 1273 Sars Cov 2 Vaccine Nejm
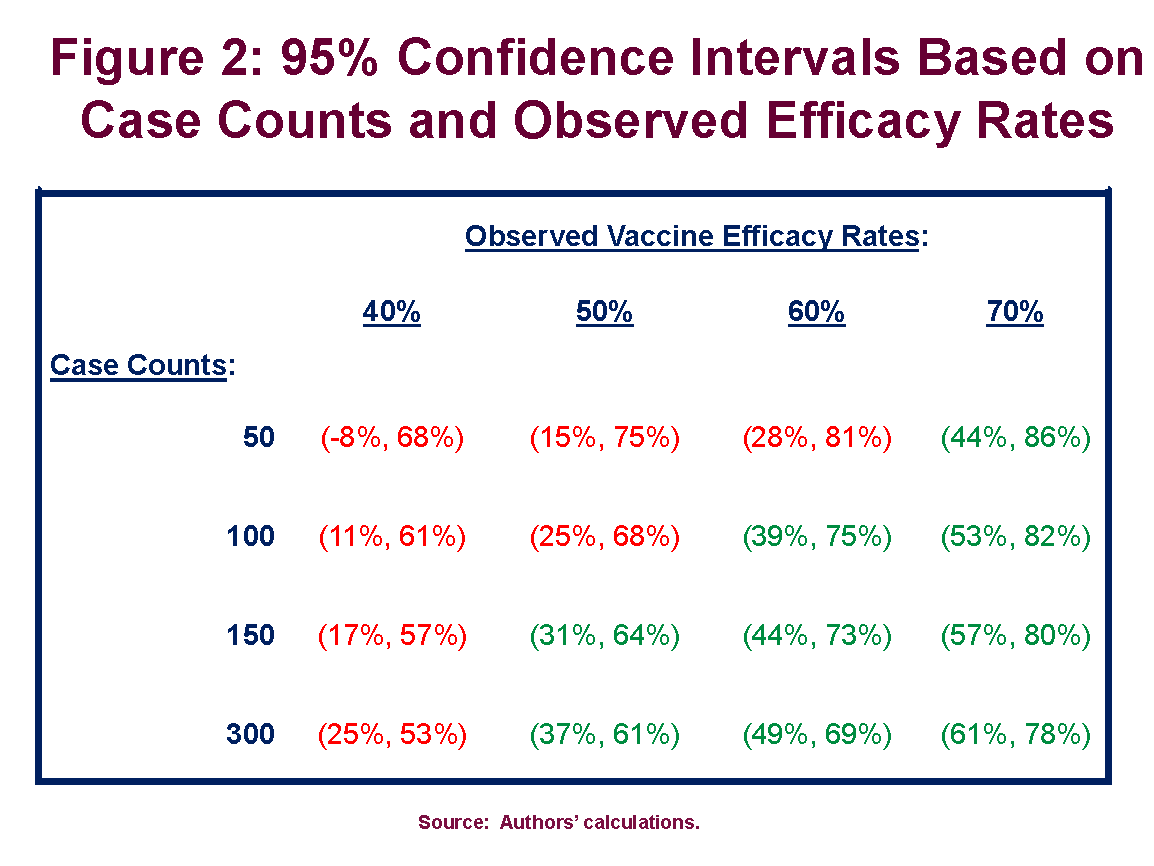 We Could Know Soon How Effective The Potential Covid Vaccines Are American Enterprise Institute Aei
We Could Know Soon How Effective The Potential Covid Vaccines Are American Enterprise Institute Aei
 Sinovac S Covid 19 Vaccine 78 Effective In Phase 3 Trial In Brazil Global Times
Sinovac S Covid 19 Vaccine 78 Effective In Phase 3 Trial In Brazil Global Times
 Oxford Vaccine Update Astrazeneca Covid 19 Vaccine Shows Promise In Elderly Trial Results By Christmas World News Times Of India
Oxford Vaccine Update Astrazeneca Covid 19 Vaccine Shows Promise In Elderly Trial Results By Christmas World News Times Of India
 No Pfizer And Novavax Vaccines Are Not The Same Here Are All Major Developers Involved In Developi The New Indian Express
No Pfizer And Novavax Vaccines Are Not The Same Here Are All Major Developers Involved In Developi The New Indian Express
Astrazeneca Manufacturing Error Clouds Vaccine Study Results The Hindu
Sinopharm S Covid 19 Vaccine Scores Approval In China Pmlive
Pfizer Claims Coronavirus Vaccine Success Plans For Emergency Ok
 What Do The Sinovac Coronavirus Vaccine Efficacy Results Mean South China Morning Post
What Do The Sinovac Coronavirus Vaccine Efficacy Results Mean South China Morning Post
 China S Covid 19 Vaccines In Demand But Will Efficacy Data Affect Its Diplomacy Goals South China Morning Post
China S Covid 19 Vaccines In Demand But Will Efficacy Data Affect Its Diplomacy Goals South China Morning Post
Https Www Alabamapublichealth Gov Covid19 Assets Cov Pfizer Moderna Vaccine Trial Summary Pdf
 First Efficacy Results Of A Covid 19 Vaccine Candidate
First Efficacy Results Of A Covid 19 Vaccine Candidate
 Moderna Raises The Bar For Covid 19 Vaccine Efficacy Evaluate
Moderna Raises The Bar For Covid 19 Vaccine Efficacy Evaluate
 Oxford Astrazeneca Vaccine May Be Trialled Again To Prove Results Were Not A Mistake
Oxford Astrazeneca Vaccine May Be Trialled Again To Prove Results Were Not A Mistake
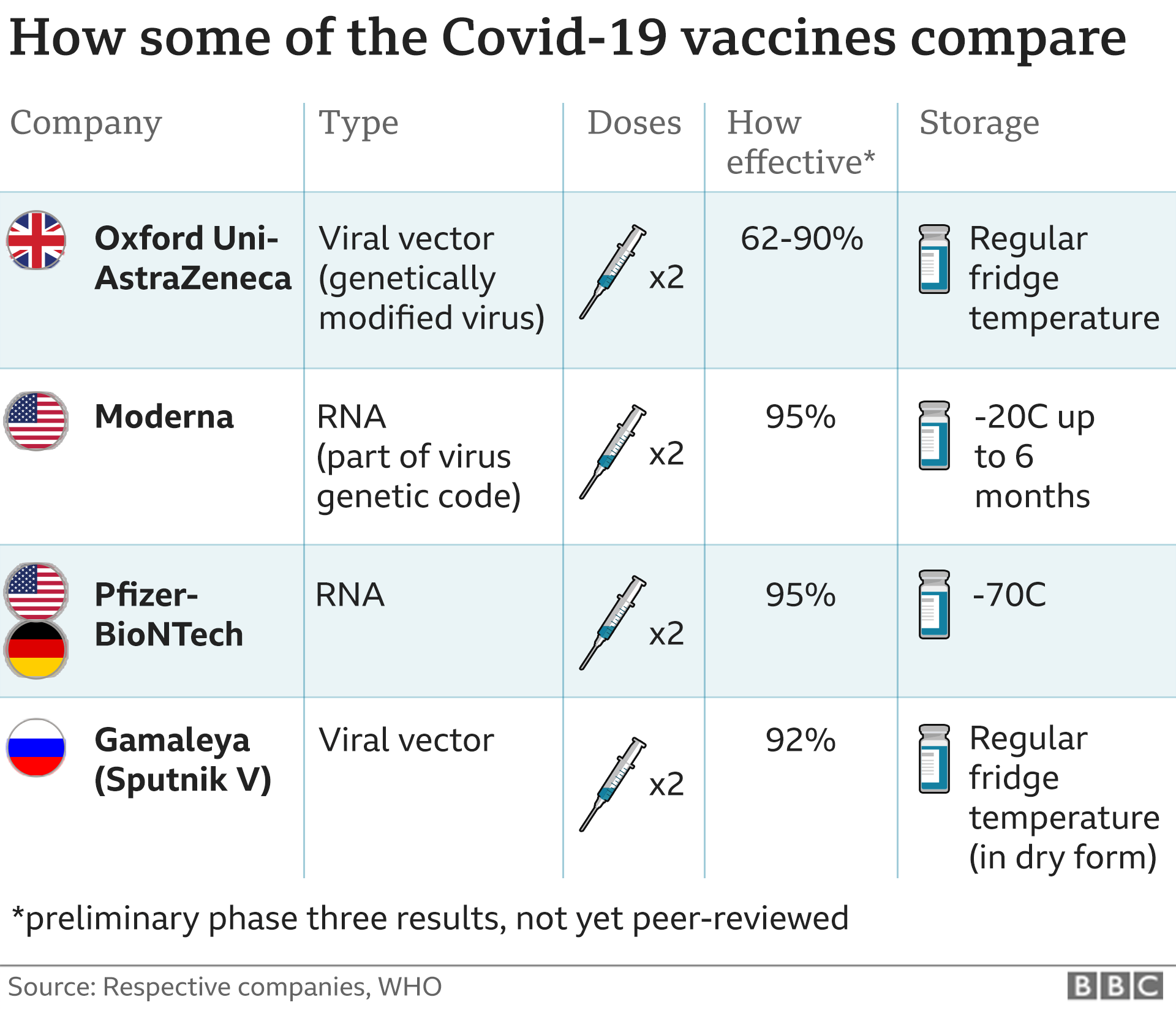 Suhu Penyimpanan Vaksin Covid Kaskus
Suhu Penyimpanan Vaksin Covid Kaskus
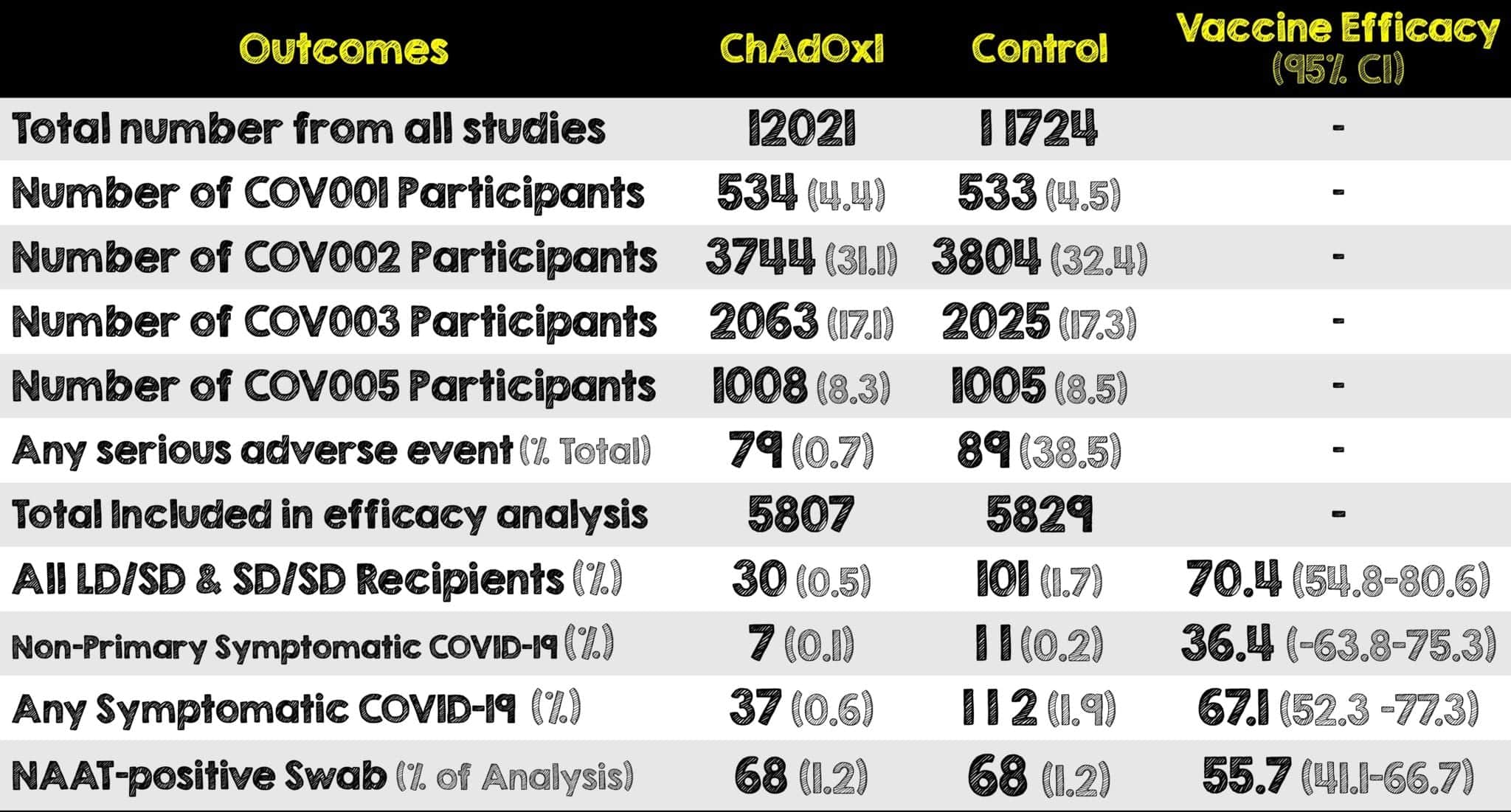 Covid 19 Update The Chadox1 Astrazeneca Covid 19 Vaccine Rebel Em Emergency Medicine Blog
Covid 19 Update The Chadox1 Astrazeneca Covid 19 Vaccine Rebel Em Emergency Medicine Blog
 Pfizer Moderna Oxford Sputnik V As Covid Vaccines Publish Results Here S What We Know
Pfizer Moderna Oxford Sputnik V As Covid Vaccines Publish Results Here S What We Know
 Coronavirus Vaccines Moderna Pfizer Sputnik Show Promising Results With 95 Efficacy Rate Youtube
Coronavirus Vaccines Moderna Pfizer Sputnik Show Promising Results With 95 Efficacy Rate Youtube
 Australia Could Be World Leader In Vaccine Production Noosa News
Australia Could Be World Leader In Vaccine Production Noosa News
 Sputnik V Covid 19 Vaccine Update Efficacy Is Over 95 42 Days After The First Dose Drug Discovery World Ddw
Sputnik V Covid 19 Vaccine Update Efficacy Is Over 95 42 Days After The First Dose Drug Discovery World Ddw
 Pfizer Reports Final Vaccine Results 95 Efficacy Ars Technica
Pfizer Reports Final Vaccine Results 95 Efficacy Ars Technica
 Peter Doshi Pfizer And Moderna S 95 Effective Vaccines We Need More Details And The Raw Data Children S Health Defense
Peter Doshi Pfizer And Moderna S 95 Effective Vaccines We Need More Details And The Raw Data Children S Health Defense
 Covid 19 Clinical Trials Recently Completed Showing Mixed Results
Covid 19 Clinical Trials Recently Completed Showing Mixed Results