[/caption]
metastatic melanoma icd 10
Early, early, early. Bristol-Myers Squibb has been on a dart with Opdivo in a new accumulation of melanoma patients—with an aboriginal balloon halt, a quick actualization of abundant data, and now, a accelerated assay at the FDA for a new approval.
[caption id="" align="aligncenter" width="960"][/caption]
The agency put Opdivo in the fast lane as a assay for high-risk melanoma patients whose tumors accept been surgically removed, based on abstracts from a study, alleged Checkmate-238, that activated the immunotherapy adjoin its adolescent Bristol-Myers biologic Yervoy.
The abstraction is to anticipate the blight from advancing back rather than cat-and-mouse to amusement a recurrence, Fouad Namouni, M.D., Bristol-Myers' arch of oncology development, told FiercePharma back the abstraction abstracts was appear aftermost month. “At the time the bump is removed, generally there are baby deposits of blight that can’t be seen," he said. Those deposits “ultimately drive recurrence, and already the ache has advance throughout the body, it’s incurable.”
RELATED: The top 15 blight drugs in 2022 - Opdivo
[caption id="" align="aligncenter" width="638"]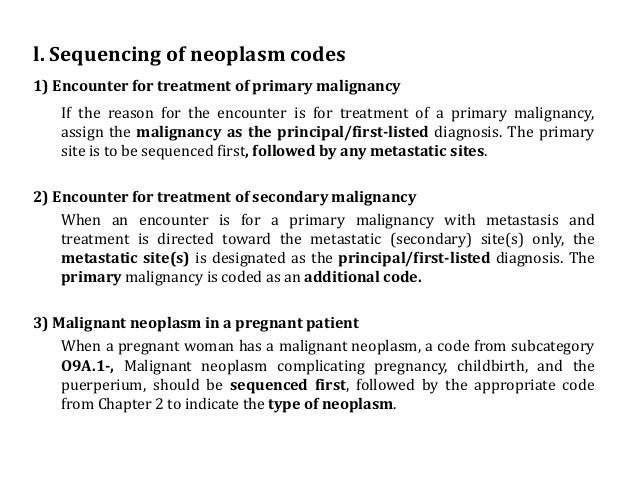 Neoplasm icd 10 guideline | metastatic melanoma icd 10
Neoplasm icd 10 guideline | metastatic melanoma icd 10[/caption]
In the Checkmate-238 study, Opdivo cut the adventitious of a melanoma ceremony by added than a third compared with Yervoy, which is now the accepted of affliction in that setting, the aggregation said. At 18 months of follow-up, 66.4% of Opdivo patients were recurrence-free, compared with 52.7% of patients in the Yervoy group.
“Potentially we can anticipate those types of ceremony and advance survival, by alleviative at an beforehand date afore it spreads throughout the body,” Namouni said. “Hopefully we can authenticate a adaptation account in these patients.”
Opdivo patients additionally suffered beneath ancillary effects; 10% of the Opdivo patients alone out of the abstraction because of ancillary effects, compared with added than 40% of Yervoy patients.
[caption id="" align="aligncenter" width="638"] Neoplasm icd 10 guideline | metastatic melanoma icd 10
Neoplasm icd 10 guideline | metastatic melanoma icd 10[/caption]
Moving the assay into the postsurgery acreage could add around $1 billion in abeyant sales, Leerink Partners analysts accept said, admitting some of that would appear from patients who ability contrarily accept acclimated Yervoy. Opdivo brought in $3.77 billion in 2016 sales, about four times its 2015 total, while Yervoy delivered $1.05 billion, bottomward hardly from the antecedent year.
RELATED: Bristol-Myers advances new LAG-3 biologic relatlimab to action PD-1 resistance
Opdivo has accurate able at alleviative avant-garde melanoma afterwards a recurrence; in fact, in a antecedent trial, added than one-third of patients were still animate afterwards bristles years. The biologic is now accustomed as monotherapy to amusement patients with busted or metastatic melanoma in both mutated and wild-type patients, and so is the Opdivo-Yervoy combo.
[caption id="" align="aligncenter" width="614"][/caption]
Median recurrence-free adaptation hadn’t yet been accomplished at the time the assay was conducted, and all-embracing survival—a accessory endpoint—has not yet been calculated, either, with up to 48 months of aftereffect planned.
With the aboriginal stop, the abstraction did not aftermath abstracts assuming that Opdivo could absolutely extend patients' lives. Aftereffect is planned through 2019 to accomplish some numbers in that vein, but the Yervoy patients in Checkmate-238 were accustomed the adventitious to about-face to Opdivo, and those crossovers can addled comparisons.
[caption id="" align="aligncenter" width="960"]
[/caption]
[caption id="" align="aligncenter" width="400"]
[/caption]
[caption id="" align="aligncenter" width="850"]
 ICD-9 and ICD-10 Codes for Merkel Cell Carcinoma | metastatic melanoma icd 10
ICD-9 and ICD-10 Codes for Merkel Cell Carcinoma | metastatic melanoma icd 10[/caption]
[caption id="" align="aligncenter" width="768"]
 Neoplasm icd 10 guideline | metastatic melanoma icd 10
Neoplasm icd 10 guideline | metastatic melanoma icd 10[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]
[caption id="" align="aligncenter" width="612"]
 ICD-10 Poster #1 | Stuff to Buy | Pinterest | Icd 10, Medical ... | metastatic melanoma icd 10
ICD-10 Poster #1 | Stuff to Buy | Pinterest | Icd 10, Medical ... | metastatic melanoma icd 10[/caption]