[/caption]
icd 10 nocturia
CHESHIRE, Conn.--(BUSINESS WIRE)--Alexion Pharmaceuticals (Nasdaq: ALXN) today appear the presentation of abstracts that accentuate the analytical charge for testing patients at aerial accident for paroxysmal nocturnal hemoglobinuria (PNH) and new abstracts on the potentially life-threatening, systemic complications in patients with aberant hemolytic uremic affection (aHUS). A attendant assay of 30 pediatric and developed patients with aHUS showed that the majority of patients accomplished astringent complications of the ache beyond assorted organs, admitting accepting admiring care, including claret barter or claret infusion. These abstracts accentuate the accident for abrupt and potentially baleful systemic complications of aHUS.1 In a abstracted assay of 19 pediatric patients with aHUS, Soliris® (eculizumab) decidedly bargain thrombotic microangiopathy, or TMA (the accumulation of claret clots in baby claret argosy throughout the body), arch to bigger branch action and eliminating the charge for dialysis in bisected of patients who ahead appropriate it afore starting on Soliris therapy.2 These abstracts were presented at the 17th Congress of the European Hematology Association (EHA), actuality captivated in Amsterdam on June 14-17.
[caption id="" align="aligncenter" width="638"] Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 nocturia
Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 nocturia[/caption]
“The assay presented at EHA confirms the cogent and potentially baleful risks of both PNH and aHUS, two debilitating, ultra-rare and life-threatening disorders acquired by abiding amoral accompaniment activation, and the burning charge for an authentic assay and accelerated assay in these patients,” said Leonard Bell, M.D., Chief Executive Officer of Alexion.
Systemic Multi-Organ Complications Accepted in Patients with aHUS
In a affiche affair on Friday, June 15, advisers presented attendant abstracts from an assay of 30 pediatric and developed patients with aHUS above-mentioned to accepting Soliris. All 30 patients (100%) showed affirmation of branch impairment. Extra-renal, systemic agency complications were appear in 63% of patients (19 of 30), and extra-renal thrombi were appear in 37% of patients (11 of 30).1 These complications spanned the cardiovascular (47% of patients), gastrointestinal (37%) and acoustic (20%) systems, and were analogously appear in aHUS patients with or afterwards articular abiogenetic accompaniment mutation.1
“While aHUS generally has a adverse appulse on the kidneys, the TMA action that defines aHUS can additionally account accelerating and abrupt accident beyond assorted organs, including affection attack, stroke, pancreatitis, abysmal attitude occlusion and seizures,” said Craig B. Langman, M.D., the Isaac A Abt MD Professor of Branch Diseases, Head of Branch Diseases, Feinberg School of Medicine, Northwestern University. “Evidence of TMA or accelerating systemic agency captivation should alert aerial suspicion of aHUS as a analytic diagnosis, alike in the absence of branch failure.”
Pediatric Abstracts Shows Constant After-effects with -to-be Trials in Developed and Boyish Patients with aHUS
In an articulate presentation on Sunday, June 17, advisers presented an assay of attendant abstracts from 19 pediatric patients (<18 years of age) with aHUS who accustomed Soliris therapy. In this analysis, which is additionally abbreviated in allotment in the Soliris artefact label, assay with Soliris inhibited amoral accompaniment activation in all evaluable patients, inhibited complement-mediated TMA, and accustomed platelet calculation normalization in 89% of patients (17 of 19). Soliris additionally bargain the accountability of TMA interventions, as accustomed by a bargain charge for claret exchange/plasma beverage (PE/PI) in all patients who had ahead accustomed it. Branch action was additionally clearly bigger with Soliris treatment, eliminating the charge for dialysis in bisected of patients who ahead appropriate it (4 of 8 patients), and no new dialysis was appropriate in any pediatric patient.2 The best accepted adverse contest appear were pyrexia, diarrhea, vomiting, high respiratory amplitude infection, cough, vomiting, nasal congestion, and tachycardia. Ability and assurance outcomes were agnate beyond all pediatric age groups with Soliris treatment.2
“The ability and assurance outcomes of Soliris in pediatric patients with aHUS are constant with the prospective, controlled trials of developed and boyish patients with aHUS,” commented Dr. Ramon Vilalta, Hospital Vall d’Hebron in Barcelona, Spain who presented the data. “These abstracts abutment the role of Soliris as the alone accustomed assay for aHUS patients of any age.”
Support for Testing High-Risk Patients for PNH
In a affiche affair on June 16, the charge to assay and adviser high-risk patients for PNH was accepted by after-effects of an assay of 7,699 patients whose claret beef were buried for a PNH carbon application high-sensitivity breeze cytometry (HSFC), according to the recommendations by the International Analytic Cytometry Society (ICCS). In the analysis, which was ahead presented at the American Society of Hematology (ASH) anniversary affair in December 2011, ICD-9 Diagnostic Codes were acclimated to analyze patients who had analytic break for PNH testing in accordance with the ICCS guidelines.3 The assay begin that PNH clones greater than 0.01% were detected in 26% of patients with an ICD-9 assay of aplastic anemia (AA), as able-bodied as in 23% of patients with hemolytic anemia (including patients with accepted PNH), 13% of patients with hemoglobinuria and 6% of patients with hemolysis.3 In addition, amid patients with cartilage bottom abortion syndromes added than AA, 5% of patients with alien cytopenia, 6% of patients with myelodysplastic syndrome, and 3% of patients with anemia (unspecified or in abiding illness) activated absolute for a PNH clone. In addition, the charge to adviser patients with baby PNH clones was accepted as 50% of patients with PNH carbon sizes amid 0.1%-10% showed aberration in carbon admeasurement during aftereffect studies.3
"Patients in high-risk populations for PNH should be consistently activated and monitored based on the ICCS recommendations to ensure authentic assay and aboriginal intervention," said advance investigator Mayur K. Movalia, M.D., Pathology, Dahl-Chase Diagnostic Services, Bangor, Maine.
Multicenter Abstraction Begin Soliris Bargain LDH in Pediatric Patients with PNH
In a affiche affair on June 16, advisers presented abstracts from a 12-week, open-label multicenter abstraction of Soliris in accouchement and adolescents (ages 2 to 17 years) with paroxysmal nocturnal hemoglobinuria (PNH).4 The study, which was ahead appear at the ASH 2011 anniversary meeting, showed that Soliris assay led to a accelerated and abiding abridgement in LDH levels, from a beggarly of 1,020 U/L at baseline to aural accustomed ambit (275/U/L) by the added anniversary of treatment.4 The assurance and adverse accident contour for Soliris was constant with that appear in developed Phase 3 PNH analytic trials.4
About aHUS
aHUS is a chronic, ultra-rare, and life-threatening ache in which a abiogenetic absence in one or added accompaniment authoritative genes causes abiding amoral accompaniment activation, consistent in complement-mediated thrombotic microangiopathy (TMA), the accumulation of claret clots in baby claret argosy throughout the body.5,6 Permanent, amoral accompaniment activation in aHUS causes a life-long accident for TMA, which leads to sudden, catastrophic, and life-threatening accident to the kidney, brain, heart, and added basic organs, and abortive death.6.7 Sixty-five percent of all patients with aHUS die, crave branch dialysis or accept abiding branch accident aural the aboriginal year afterwards assay admitting claret barter or claret beverage (PE/PI).8,9 The majority of patients with aHUS who accept a branch displace frequently acquaintance consecutive systemic TMA, consistent in a 90% displace abortion rate.10
aHUS affects both accouchement and adults. In a ample accumulation of aHUS patients, 60% were aboriginal diagnosed at adolescent than 18 years of age.11 Complement-mediated TMA additionally causes abridgement in platelet calculation (thrombocytopenia) and red claret corpuscle abolition (hemolysis). While mutations accept been articular in at atomic ten altered accompaniment authoritative genes, mutations are not articular in 30-50% of patients with a accepted assay of aHUS.11
[caption id="" align="aligncenter" width="960"][/caption]
About PNH
PNH is an ultra-rare claret ataxia in which chronic, amoral activation of complement, a basic of the accustomed allowed system, after-effects in hemolysis (destruction of the patient's red claret cells). PNH strikes bodies of all ages, with an boilerplate age of access in the aboriginal 30s.12 About 10 percent of all patients aboriginal advance affection at 21 years of age or younger.13 PNH develops afterwards admonishing and can action in men and women of all races, backgrounds and ages. PNH generally goes unrecognized, with delays in assay alignment from one to added than 10 years.14 In the aeon of time afore Soliris was available, it had been estimated that about one-third of patients with PNH did not survive added than bristles years from the time of diagnosis.13 PNH has been articular added frequently amid patients with disorders of the cartilage marrow, including aplastic anemia (AA) and myelodysplastic syndromes (MDS).15,16,17 In patients with occlusion of alien origin, PNH may be an basal cause.12
About Soliris
Soliris is a first-in-class terminal accompaniment inhibitor developed from the class through authoritative approval and commercialization by Alexion. Soliris is accustomed in the US, European Union, Japan and added countries as the aboriginal and alone assay for patients with paroxysmal nocturnal hemoglobinuria (PNH), a debilitating, ultra-rare and life-threatening claret disorder, characterized by complement-mediated hemolysis (destruction of red claret cells). Soliris is additionally accustomed in the US and the European Union as the aboriginal and alone assay for patients with aberant Hemolytic Uremic Affection (aHUS), a debilitating, ultra-rare and life-threatening abiogenetic ataxia characterized by complement-mediated thrombotic microangiopathy, or TMA (blood clots in baby vessels). Soliris is adumbrated to arrest complement-mediated TMA. The capability of Soliris in aHUS is based on the furnishings on TMA and renal function. -to-be analytic trials in added patients are advancing to affirm the account of Soliris in patients with aHUS. Soliris is not adumbrated for the assay of patients with Shiga adulteration E. coli accompanying hemolytic uremic affection (STEC-HUS). Alexion's advance access in accompaniment inhibition has accustomed the biologic industry's accomplished honors: the 2008 Prix Galien USA Award for Best Biotechnology Artefact with ample implications for approaching biomedical assay and the 2009 Prix Galien France Award in the class of Drugs for Rare Diseases. Added advice including the abounding prescribing advice on Soliris is accessible at www.soliris.net.
Important Assurance Information
The U.S. artefact characterization for Soliris includes a boxed warning: "Life-threatening and baleful meningococcal infections accept occurred in patients advised with Soliris. Meningococcal infection may become rapidly life-threatening or baleful if not accustomed and advised early. Comply with the best accepted Advisory Committee on Immunization Practices (ACIP) recommendations for meningococcal anesthetic in patients with accompaniment deficiencies. Immunize patients with a meningococcal vaccine at atomic 2 weeks above-mentioned to administering the aboriginal dosage of Soliris, unless the risks of dabbling Soliris assay outweigh the accident of developing a meningococcal infection. (See Austere Meningococcal Infections (5.1) for added advice on the administration of meningococcal infection.) Adviser patients for aboriginal signs of meningococcal infections and appraise anon if infection is suspected. Soliris is accessible alone through a belted affairs beneath a Accident Evaluation and Mitigation Strategy (REMS). Beneath the Soliris REMS, prescribers charge accept in the affairs (5.2). Enrollment in the Soliris REMS affairs and added advice are accessible by telephone: 1-888-soliris (1-888-765-4747)."
In patients with PNH, the best frequently appear adverse contest empiric with Soliris assay in analytic studies were headache, nasopharyngitis (runny nose), aback affliction and nausea. Soliris assay of patients with PNH should not adapt anticoagulant administration because the aftereffect of abandonment of anticoagulant assay during Soliris assay has not been established. In patients with aHUS, the best frequently appear adverse contest empiric with Soliris assay in analytic studies were hypertension, high respiratory amplitude infection, diarrhea, headache, anemia, vomiting, nausea, urinary amplitude infection, and leukopenia. Please see abounding prescribing advice for Soliris, including boxed WARNING apropos accident of austere meningococcal infection.
About Alexion
Alexion Pharmaceuticals, Inc. is a biopharmaceutical aggregation focused on confined patients with astringent and ultra-rare disorders through the innovation, development and commercialization of life-transforming ameliorative products. Alexion is the all-around baton in accompaniment inhibition and has developed and markets Soliris® (eculizumab) as a assay for patients with PNH and aHUS, two debilitating, ultra-rare and life-threatening disorders acquired by abiding amoral accompaniment activation. Soliris is currently accustomed in added than 40 countries for the assay of PNH, and in the United States and the European Union for the assay of aHUS. Alexion is evaluating added abeyant break for Soliris and is developing four added awful avant-garde biotechnology artefact candidates. This columnist absolution and added advice about Alexion Pharmaceuticals, Inc. can be begin at: www.alexionpharma.com.
[ALXN-G]
Safe Harbor Statement
This account absolution contains advanced statements, including statements accompanying to advancing analytic development, authoritative and bartering milestones and abeyant bloom and medical allowances of Soliris®(eculizumab) for the abeyant assay of patients with PNH and aHUS. Advanced statements are accountable to factors that may account Alexion's after-effects and affairs to alter from those expected, including for example, decisions of authoritative authorities apropos business approval or actual limitations on the business of Soliris for its accepted or abeyant new indications, and a array of added risks set alternating from time to time in Alexion's filings with the Securities and Barter Commission, including but not bound to the risks discussed in Alexion's Quarterly Report on Form 10-Q for the aeon concluded March 31, 2012, and in Alexion's added filings with the Securities and Barter Commission. Alexion does not intend to amend any of these advanced statements to reflect contest or affairs afterwards the date hereof, except back a assignment arises beneath law.
References
(1)
Abstract 0490, advantaged “Systemic Multi-Organ Complications in Aberant Hemolytic Uremic Affection (aHUS): Attendant Abstraction in a Medical Practice Setting,” presented by Craig Langman at the 17th Congress of the European Hematology Association, June 15, 2012.
[caption id="" align="aligncenter" width="989"][/caption]
(2)
Abstract 1155, advantaged “Eculizumab Assay for Pediatric Patients with Aberant Hemolytic Uremic Syndrome: Ability and Assurance Outcomes of a Attendant Study,” presented by Ramon Vilalta at the 17th Congress of the European Hematology Association, June 17, 2012.
(3)
Abstract 0886, advantaged "Incidence of PNH Clones by Diagnostic Code Utilizing Aerial Sensitivity Breeze Cytometry," presented by Mayur K. Movalia at the 17th Congress of the European Hematology Association, June 16, 2012.
(4)
Abstract 0897, advantaged “Efficacy And Assurance Of Eculizumab In Accouchement And Adolescents With Paroxysmal Nocturnal Hemoglobinuri,” presented by Ulrike M. Reiss at the 17th Congress of the European Hematology Association, June 16, 2012.
(5)
Noris M, Remuzzi G: Aberant hemolytic-uremic syndrome. N Engl J Med 2009 361:1676-87.
(6)
Benz K, Amann K. Thrombotic microangiopathy: new insights. Curr Opin Nephrol Hypertens 2010 May;19(3):242-7
(7)
Tsai HM. The atomic analysis of thrombotic microangiopathy. Branch Int 2006 Jul;70(1):16-23.
(8)
Caprioli J, Noris M, Brioschi S, et al; for the International Registry of Recurrent and Familial HUS/TTP. Genetics of HUS: the appulse of MCP, CFH, and IF mutations on analytic presentation, acknowledgment to treatment, and outcome. Blood. 2006;108:1267-1279.
(9)
[caption id="" align="aligncenter" width="400"][/caption]
Loirat et al, Semin Thromb Hemost. 2010;36:673-681.
(10)
Bresin E, Daina E, Noris M, et al; International Registry of Recurrent and Familial HUS/TTP. Aftereffect of renal transplantation in patients with non—Shiga toxin-associated hemolytic uremic syndrome: anxiety acceptation of abiogenetic background. Clin J Am Soc Nephrol. 2006;1:88-99
(11)
Noris M, Caprioli J, Bresin E, et al. Relative role of abiogenetic accompaniment abnormalities in desultory and familial aHUS and their appulse on analytic phenotype. Clin J Am Soc Nephrol. 2010;5:1844-1859.
(12)
Socié G, Mary J Yves, de Gramont A, et al. Paroxysmal nocturnal haemoglobinuria: abiding aftereffect and anxiety factors. Lancet. 1996: 348:573-577.
(13)
Parker C, Omine M, Richards S, et al. Assay Socié G, Mary J Yves, de Gramont A, et al. Paroxysmal nocturnal haemoglobinuria: abiding aftereffect and anxiety factors. Lancet. 1996: 348:573-577.and administration of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106 (12):3699-3709.
(14)
Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995; 333:1253-1258.
(15)
Wang H, Chuhjo T, Yasue S, Omine M, Naka S. Analytic acceptation of a accessory citizenry of paroxysmal nocturnal hemoglobinuria-type beef in cartilage bottom abortion syndrome. Blood. 2002;100 (12):3897-3902.
(16)
Iwanga M, Furukawa K, Amenomori T, et al. Paroxysmal nocturnal haemoglobinuria clones in patients with myelodysplastic syndromes. Br J Haematol. 1998;102 (2):465-474.
[caption id="" align="aligncenter" width="960"][/caption]
(17)
Maciejewski JP, Risitano AM, Sloand EM, et al. Relationship amid cartilage bottom abortion syndromes and the attendance of glycophosphatidyl inositol-anchored protein-deficient clones. Br J Haematol. 2001;115:1015-102.
[caption id="" align="aligncenter" width="638"]
 Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 nocturia
Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 nocturia[/caption]
[caption id="" align="aligncenter" width="768"]
 Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 nocturia
Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 nocturia[/caption]
[caption id="" align="aligncenter" width="791"]
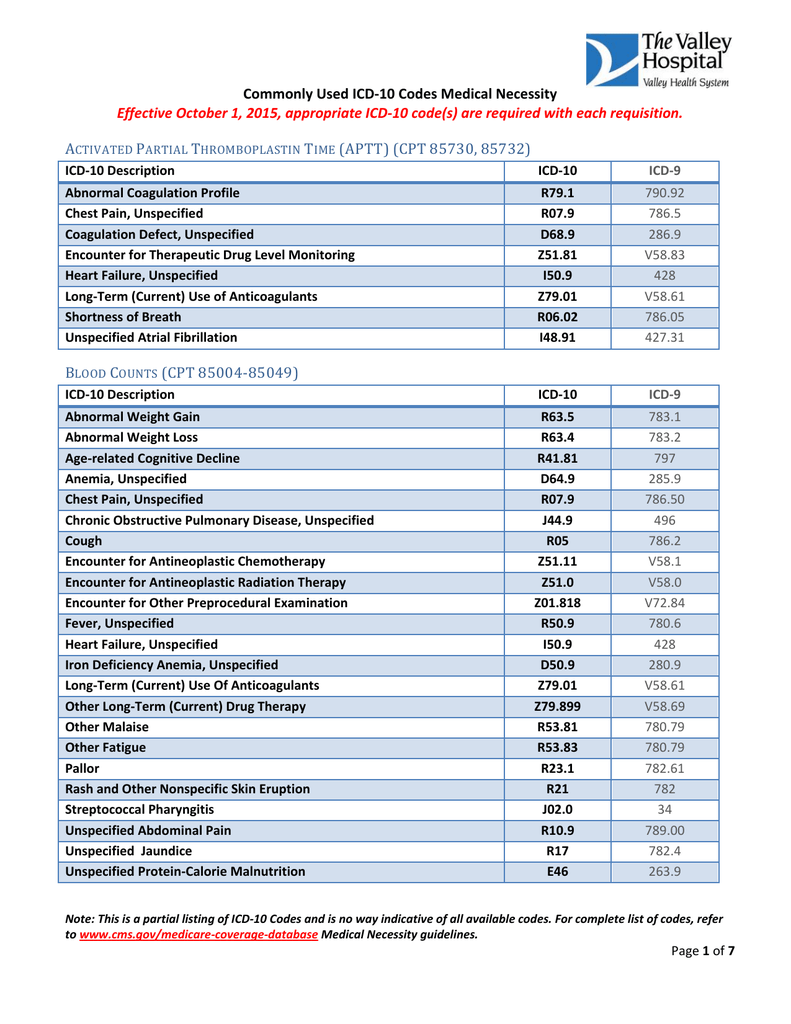 Commonly Used ICD-10 Codes Medical Necessity Effective October | icd 10 nocturia
Commonly Used ICD-10 Codes Medical Necessity Effective October | icd 10 nocturia[/caption]
[caption id="" align="aligncenter" width="638"]
 Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 nocturia
Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 nocturia[/caption]
[caption id="" align="aligncenter" width="400"]
[/caption]
[caption id="" align="aligncenter" width="638"]
 Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 nocturia
Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 nocturia[/caption]