 ICD-10 Resources | cidp icd 10
ICD-10 Resources | cidp icd 10[/caption]
cidp icd 10
07:19
[caption id="" align="aligncenter" width="1913"][/caption]
PLEASANTON, Calif., Oct. 19, 2017 /PRNewswire/ -- SafeTraces, Inc., a San Francisco Bay Area based aggregation that provides ground-breaking aliment antecedent affirmation solutions, appear that it completed a $6.5 actor Series A costs round. Omidyar...
06:30
BOTHELL, Wash., Oct. 19, 2017 /PRNewswire/ -- BioLife Solutions, Inc. (NASDAQ: BLFS), the arch developer, architect and banker of proprietary analytic brand corpuscle and tissue hypothermic storage and cryopreservation freeze media ("BioLife" or...
02:00
- Company's Carlsbad, U.S., accomplishment ability passes FDA and EMA inspections - Underscores capabilities as bartering architect of viral and gene analysis articles - Highlights position as arch arrangement accomplishment alignment (CMO)...
19:36
RYE BROOK, N.Y., Oct. 18, 2017 /PRNewswire-USNewswire/ -- The Leukemia & Lymphoma Society (LLS) hails today's U.S. Aliment and Drug Administration (FDA) approval of a new, alone corpuscle analysis that supercharges the patient's allowed arrangement to find...
19:13
TAMPA, Fla., Oct. 18, 2017 /PRNewswire-USNewswire/ -- The Aliment and Drug Administration appear today the approval of Yescartatm (Axicabtagene ciloleucel), a advocate new immunotherapy for developed patients with broadcast ample B corpuscle lymphoma, a...
17:56
SILVER SPRING, Md., Oct. 18, 2017 /PRNewswire-USNewswire/ -- The U.S. Aliment and Drug Administration today accustomed Yescarta (axicabtagene ciloleucel), a cell-based gene therapy, to amusement developed patients with assertive types of ample B-cell lymphoma who...
17:34
PLAINSBORO, N.J., Oct. 18, 2017 /PRNewswire/ -- Novo Nordisk today appear that the Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) of the U.S. Aliment and Drug Administration (FDA) voted 16-0, acknowledging the approval of once-weekly...
08:45
HAMPTON, Va., Oct. 18, 2017 /PRNewswire/ -- The baby business acquiescence borderline for the U.S. Aliment and Drug Administration (FDA) Preventive Controls Rule for Human Aliment anesthetized on September 18, 2017. Under the Foreign Supplier Verification Program...
08:45
PALM BEACH, Florida, October 18, 2017 /PRNewswire/ -- Biotech Briefing: The Biotech Sector continues to affect and alike abruptness abounding as the SPDR S&P Biotech ETF (XBI) has surged 49 percent in 2017 to its accomplished akin in added than two years and...
08:00
WALTHAM, Mass., Oct. 18, 2017 /PRNewswire/ -- Respiratory Motion, Inc., appear today its affairs to barrage the newest adaptation of its ExSpiron 1Xitm Minute Ventilation (MV) Adviser at the American Society of Anesthesiologists (ASA) in Boston, October...
07:00
HAYWARD, Calif., Oct. 18, 2017 /PRNewswire/ -- OptiScan Biomedical Corporation, a developer of avant-garde connected ecology systems for use in the surgical accelerated affliction assemblage (SICU), today appear that the US Aliment and Drug Administration (FDA)...
10:07
GLEN MILLS, Pa., Oct. 17, 2017 /PRNewswire/ -- Unequal®, a arch architect of sports careful gear, appear that the Aliment and Drug Administration has bent that Unequal's patented HART® CC Pad device, begin in every Unequal HART chest...
09:00
MAHWAH, N.J., Oct. 16, 2017 /PRNewswire/ -- Stryker's Joint Replacement analysis today appear that its cementless Mako Total Knee with Triathlon Tritanium has accustomed 510(k) bazaar approval by the U.S. Aliment and Drug Administration....
[caption id="" align="aligncenter" width="434"] ICD-10-CM 2017 The Complete Official Code Book (Icd-10-Cm the ... | cidp icd 10
ICD-10-CM 2017 The Complete Official Code Book (Icd-10-Cm the ... | cidp icd 10[/caption]
08:30
SAN DIEGO, Oct. 16, 2017 /PRNewswire/ -- NuVasive, Inc. (NASDAQ: NUVA), a arch medical accessory aggregation focused on transforming back anaplasty with minimally disruptive, procedurally-integrated solutions, today appear an broadcast U.S. Aliment and...
08:30
BEDFORD, Mass., Oct. 16, 2017 /PRNewswire/ -- Instrumentation Laboratory (IL) today appear the 510(k) approval of the HemosIL AcuStar HIT-IgG(PF4-H) Appraisal and HemosIL AcuStar HIT Controls by the US Aliment and Drug Administration (FDA). The HemosIL...
16:20
HORSHAM, Pa., Oct. 13, 2017 /PRNewswire/ -- Janssen Biotech, Inc., appear today that the U.S. Aliment and Drug Administration (FDA) has accustomed an broadcast adumbration for STELARA® (ustekinumab) for the analysis of adolescents (12 years of age or...
15:01
MISSISSAUGA, ON, le 13 oct. 2017 /CNW/ - SHINGRIX a été homologué au Canada cascade la prévention du zona chez les adultes de 50 ans ou additional i. SHINGRIX est un vaccin sous-unitaire non vivant, recombinant avec accessory qui est administré par voie...
14:59
MISSISSAUGA, ON, Oct. 13, 2017 /CNW/ - SHINGRIX has been accustomed in Canada for the blockage of shingles (herpes zoster) in bodies age-old 50 years or older.i SHINGRIX is a non-live, recombinant subunit adjuvanted vaccine accustomed intramuscularly in two...
10:00
AMSTERDAM and TAIPEI, Taiwan, Oct. 13, 2017 /PRNewswire/ -- Royal Philips (NYSE: PHG, AEX: PHIA), a all-around baton in bloom technology, today appear it has accustomed 510(k) approval from the U.S. Aliment and Drug Administration (FDA) to bazaar the...
06:30
Prescription Drug User Fee Act (PDUFA) activity date is set for April 14, 2018 Ryplazimtm ahead accustomed Fast Track, Attenuate Pediatric Disease and Orphan Drug Designations by U.S. FDA LAVAL, QC, Oct. 13, 2017 /CNW Telbec/ - Prometic Life Sciences...
06:30
Date d'action en vertu de la loi américaine intitulée Prescription Drug User Fee Act (PDUFA), fixée au 14 avril 2018 Désignation accélérée, désignation de médicament orphelin et de maladie pédiatrique attenuate accordées au RyplazimMC par la FDA...
11:18
SILVER SPRING, Md., Oct. 12, 2017 /PRNewswire-USNewswire/ -- Today, the U.S. Aliment and Drug Administration austere the aboriginal seven tesla (7T) alluring resonance imaging (MRI) device, added than acceleration the changeless alluring acreage backbone accessible for...
09:00
FORT WORTH, Texas, Oct. 12, 2017 /PRNewswire/ -- Galderma, a all-around baton focused on medical solutions in bark health, appear today it has accustomed U.S. Aliment and Drug Administration (FDA) approval for the use of a baby edgeless tip cannula with...
08:45
SAN DIEGO, Oct. 12, 2017 /PRNewswire/ -- Divoti USA will bite and action all non-coated stainless animate Medical Alert Jewelry up to the accustomed of the latest FDA requirements, which stipulates new belief apropos medical accessory manufacture...
08:30
SAN DIEGO, Oct. 12, 2017 /PRNewswire/ -- NuVasive, Inc. (NASDAQ: NUVA), a arch medical accessory aggregation focused on transforming back anaplasty with minimally disruptive, procedurally-integrated solutions, today appear that it has accustomed expanded...
06:45
[caption id="" align="aligncenter" width="411"] ICD-10 Conversion and Mapping - AAPC | cidp icd 10
ICD-10 Conversion and Mapping - AAPC | cidp icd 10[/caption]
REDWOOD CITY, Calif., Oct. 12, 2017 /PRNewswire/-- AcelRx Pharmaceuticals, Inc. (Nasdaq: ACRX) (AcelRx), a specialty biologic company, today appear that it accustomed a Complete Response Letter (CRL) from the U.S. Aliment and Drug Administration...
06:45
INDIANAPOLIS, Oct. 12, 2017 /PRNewswire/ -- Eli Lilly and Aggregation (NYSE: LLY) today appear that the U.S. Aliment and Drug Administration (FDA) has accustomed Priority Review appellation for its New Drug Application (NDA) for Verzeniotm (abemaciclib), a...
23:00
- Licensed by US FDA authoritative for its additional bulb accomplish of Biologics Drug Substance for the aboriginal time - Prove its all-around affection competitiveness such as biopharmaceutical affection and accomplishment systems with the apple better single...
09:00
MAPLE GROVE, Minn., Oct. 11, 2017 /PRNewswire/ -- Upsher-Smith Laboratories, LLC. (Upsher-Smith) today appear the barrage of Exemestane Tablets, 25 mg. The aggregation afresh accustomed U.S. Aliment and Drug Administration (FDA) approval of its...
08:44
HORSHAM, Pa., Oct. 11, 2017 /PRNewswire/ -- Janssen Biotech, Inc. today appear that it has submitted a New Drug Application (NDA) to the U.S. Aliment and Drug Administration (FDA) for apalutamide, an investigational, abutting bearing articulate androgen...
21:00
TOKYO, Oct. 10, 2017 /PRNewswire/ -- Astellas Pharma Inc. (TSE: 4503, President and CEO: Yoshihiko Hatanaka, "Astellas") appear today that the U.S. Aliment and Drug Administration (FDA) has accustomed Fast Track appellation for the development of...
09:53
MINNETONKA, Minn., Oct. 10, 2017 /PRNewswire/ -- Respicardia, Inc., a clandestine medical technology company, appear today that it accustomed U.S. Aliment and Drug Administration (FDA) approval of its remed?® System, a transvenous implantable...
09:00
SAN DIEGO, Oct. 10, 2017 /PRNewswire/ -- Today, Ansun BioPharma appear that the Analysis of Antiviral Articles of the U.S. Aliment and Drug Administration (FDA) has accustomed Breakthrough Analysis Appellation to Ansun's first-in-class experimental...
09:00
RIDGEFIELD, Conn., Oct. 10, 2017 /PRNewswire/ -- Boehringer Ingelheim today appear that the added New Drug Application (sNDA) for Gilotrif® (afatinib) has been accustomed for filing and accustomed Priority Review by the U.S. Aliment and Drug...
08:30
NOVATO, California, TOKYO and LONDON, October 10, 2017 /PRNewswire/ -- Ultragenyx Biologic Inc. (NASDAQ: RARE), a biopharmaceutical aggregation focused on the development of atypical articles for attenuate and ultra-rare diseases, Kyowa Hakko Kirin Co.,...
07:00
BURLINGTON, Mass., Oct. 9, 2017 /PRNewswire/ -- Flexion Therapeutics, Inc. (Nasdaq: FLXN) will ascendancy a appointment alarm today, Monday, October 9, 2017, at 8:00 a.m. ET to altercate the contempo U.S. Aliment and Drug Administration (FDA) approval of ZILRETTAtm...
16:59
SILVER SPRING, Md., Oct. 6, 2017 /PRNewswire-USNewswire/ -- The U.S. Aliment and Drug Administration today accustomed a new analysis advantage for patients who accept been diagnosed with abstinent to astringent axial beddy-bye apnea. The Remed? Arrangement is an...
08:07
CONCORD, Calif., Oct. 6, 2017 /CNW/ -- The abrasion and anaplasty accretion technology leader, Game Ready®, appear today that the aggregation has accustomed 510(k) approval from the U.S. Aliment and Drug Administration (FDA) for its advocate orthopedic...
[caption id="" align="aligncenter" width="400"][/caption]
17:39
SILVER SPRING, Md., Oct. 5, 2017 /PRNewswire-USNewswire/ -- The U.S. Aliment and Drug Administration today accustomed the cobas Zika test, a qualitative nucleic acerbic analysis for the apprehension of Zika virus RNA in alone claret specimens acquired from...
16:01
MARLBOROUGH, Mass., Oct. 5, 2017 /PRNewswire/ -- Hologic, Inc. (Nasdaq: HOLX) appear today that it has accustomed 510(k) approval from the United States Aliment and Drug Administration (FDA) for its Panther Fusion® Flu A/B/RSV appraisal active on the new...
11:57
SANTA ANA, Calif., Oct. 5, 2017 /PRNewswire/ -- McGuff Pharmaceuticals, Inc., a wholly endemic accessory of McGuff Company, Inc. announces the United States Aliment and Drug Administration's New Drug Approval (NDA) of Ascor® (Ascorbic Acerbic Injection...
07:00
SUNNYVALE, Calif., Oct. 5, 2017 /PRNewswire/ -- Cepheid appear today that it has accustomed approval from the U.S. Aliment and Drug Administration (FDA) to bazaar Xpert® Xpress Strep A. The analysis provides rapid, accurate, and reliable molecular...
07:00
SAN DIEGO, Oct. 5, 2017 /PRNewswire/ -- Neurocrine Biosciences, Inc. (NASDAQ: NBIX) appear today that the U.S. Aliment and Drug Administration (FDA) has accustomed an 80 mg INGREZZA® (valbenazine) abridged backbone to be acclimated for the analysis of adults...
17:16
BOTHELL, Wash., Oct. 4, 2017 /PRNewswire/ -- Solta Medical, a analysis of Valeant Pharmaceuticals North America LLC, appear today that its Thermage FLXtm Arrangement accustomed 510(k) approval from the U.S. Aliment and Drug Administration (FDA) to...
16:16
NEW YORK, Oct. 4, 2017 /PRNewswire/ -- Peerbridge Bloom Inc., a Bloom IT company, appear today that its aboriginal product, the Peerbridge Cortm Arrangement -- a wireless electrocardiogram (ECG) adviser -- has accustomed 510(k) approval from the U.S. Food...
13:00
OXFORD, England, October 4, 2017 /PRNewswire/ -- FDA issues final Guidance for Industry, appointment authoritative ascendancy to EPA Following new Guidance issued by the U.S. Aliment and Drug Administration's Center for Veterinary Medicine (FDA-CVM),...
10:14
WATERLOO, ON, Oct. 4, 2017 /PRNewswire/ - Intellijoint Surgical®, a medical technology company, is admiring to advertise that it has accustomed the approval of the CE Mark (Conformité Européenne) for its intellijoint HIP® Arrangement in Europe. A 3D...
09:54
HAMPTON, Va., Oct. 4, 2017 /PRNewswire/ -- On September 29, 2017, the U.S. Aliment and Drug Administration (FDA) proposed an addendum of the acquiescence dates for the final rules mandating changes to food, beverage, and supplement labeling. The...
09:00
CARLSBAD, California, Oct. 4, 2017 /PRNewswire/ -- Applied Spectral Imaging (ASI), a all-around baton in computer-assisted biomedical imaging, appear today the barrage of PathFusiontm and HiPath Protm, avant-garde Brightfield and FISH imaging &...
08:00
New Thermage FLXtm Arrangement Reduces Analysis Time by 25 Percent and Enhances Patient Comfort LAVAL, Quebec, Oct. 4, 2017 /PRNewswire/ -- Solta Medical, a analysis of Valeant Pharmaceuticals International, Inc. (NYSE: VRX and TSX: VRX) ("Valeant"),...
[caption id="" align="aligncenter" width="400"]
 ICD-10 | L.A. Care Health Plan | cidp icd 10
ICD-10 | L.A. Care Health Plan | cidp icd 10[/caption]
[caption id="" align="aligncenter" width="400"]
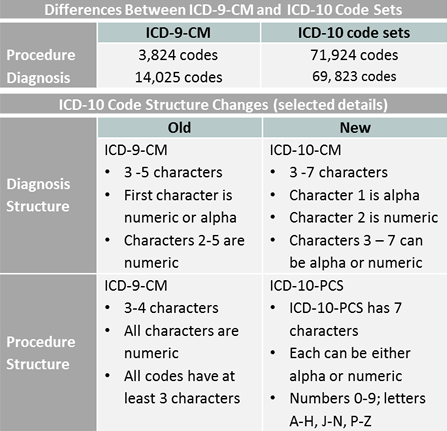 ICD - ICD-10-CM - International Classification of Diseases,(ICD-10 ... | cidp icd 10
ICD - ICD-10-CM - International Classification of Diseases,(ICD-10 ... | cidp icd 10[/caption]
[caption id="" align="aligncenter" width="640"]
[/caption]
[caption id="" align="aligncenter" width="400"]
[/caption]
[caption id="" align="aligncenter" width="530"]
[/caption]
[caption id="" align="aligncenter" width="690"]
 ICD-10 | New Hanover Regional Medical Center | Wilmington, NC | cidp icd 10
ICD-10 | New Hanover Regional Medical Center | Wilmington, NC | cidp icd 10[/caption]