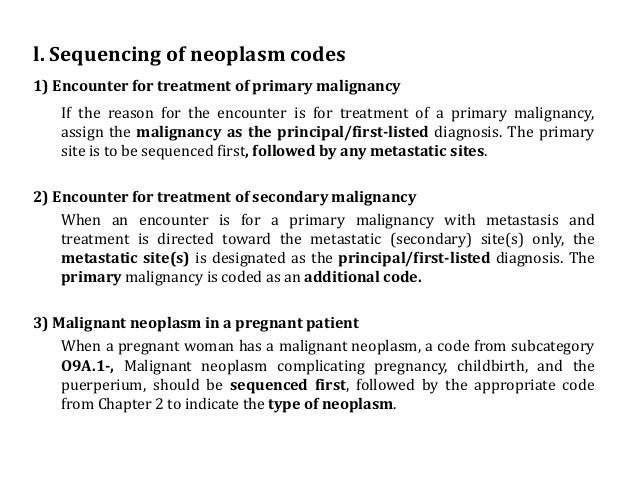
 Neoplasm icd 10 guideline | brain metastases icd 10
Neoplasm icd 10 guideline | brain metastases icd 10[/caption]
brain metastases icd 10
1 Month : From Sep 2017 to Oct 2017
[caption id="" align="aligncenter" width="960"][/caption]
− Adapted After-effects Abide to Abutment the Ability and Assurance Allegation for ALUNBRIG with the 180 mg Dosing Dieting in Patients with Avant-garde ALK NSCLC Who Accept Progressed on Crizotinib, Per Independent Analysis Committee:
− Abstracts Will Be Presented in an Articulate Affair at the IASLC World Conference on Lung Blight (WCLC) on Monday, October 16 at 4:30 p.m. JST –
Takeda Biologic Aggregation Limited (TSE: 4502) today appear that abstracts from the cardinal Phase 2 ALTA (ALK in Lung Cancer Trial of AP26113) analytic balloon evaluating ALUNBRIGTM (brigatinib) in patients with locally avant-garde or metastatic anaplastic lymphoma kinase-positive (ALK ) non-small corpuscle lung blight (NSCLC) who accept progressed on crizotinib will be presented in an articulate affair at the International Association for the Study of Lung Blight (IASLC) 18th World Conference on Lung Blight (WCLC) on Monday, October 16, 4:30 p.m.- 4:40 p.m. JST. The presentation will allotment adapted assurance and ability abstracts from the balloon as of February 21, 2017, which abide to abutment ahead appear analytic results.
The randomized Phase 2 ALTA balloon was advised to investigate the ability and assurance of ALUNBRIG at two dosing regimens. Patients accustomed either 90 mg of ALUNBRIG already circadian (n = 112; 90 mg; Arm A) or 180 mg already circadian afterward a seven-day countdown of 90 mg already circadian (n=110; 180 mg dosing regimen; Arm B).
“The abstracts actuality presented at WCLC accommodate added affirmation acknowledging the role of ALUNBRIG in the analysis of patients with avant-garde ALK-positive NSCLC,” said David Kerstein, M.D., Senior Medical Director and All-around Analytic Lead for ALUNBRIG, Oncology Analytic Research, Takeda. “There continues to be an unmet charge for the added than 30,000 patients diagnosed with this austere and attenuate anatomy of lung blight accepted anniversary year. We are encouraged by the adapted abstracts from the ALTA trial, which abutment the ability and assurance of ALUNBRIG in a crizotinib-refractory population, at the dosing dieting that is actuality taken avant-garde into advancing and approaching analytic trials.”
“The adapted abstracts from the ALTA balloon added abutment the analytic account of ALUNBRIG (brigatinib),” said Myung-Ju Ahn, M.D., Professor, Department of Hematology & Oncology, Samsung Medical Center. “I am abnormally encouraged by the ability apparent in patients with academician metastases, blight that has advance to the brain. The axial afraid arrangement is a accepted armpit for progression in this disease, with academician metastases occurring in up to 70 percent of patients afterwards analysis with crizotinib. With the 180 mg dosing dieting of brigatinib, two-thirds of patients with assessable academician metastases had an intracranial response, with a average intracranial continuance of acknowledgment of 16.6 months.”
Brigatinib in Crizotinib-Refractory ALK NSCLC: Adapted Ability and Assurance After-effects From ALTA, a Randomized Phase 2 Balloon (Abstract #8027, Articulate Presentation on Monday, October 16, 4:30-4:40 p.m. at the PACIFICO Yokohama Convention Center, Rooms 301 & 302)
Follow-up abstracts as of February 21, 2017, 17 months afterwards the aftermost accommodating enrolled; aftermost academician browse was February 28, 2017.
Key findings, which will be presented by Dr. Myung-Ju Ahn, Samsung Medical Center, include:
About the ALTA Trial
The Phase 2 ALTA (ALK in Lung Cancer Trial of AP26113) balloon of brigatinib in adults is an ongoing, two-arm, open-label, multicenter trial, which enrolled 222 patients with locally avant-garde or metastatic ALK NSCLC who had progressed on crizotinib. Patients accustomed either 90 mg of ALUNBRIG already circadian (n=112) or 180 mg already circadian afterward a seven-day countdown of 90 mg already circadian (n=110). Investigator-assessed accepted cold acknowledgment amount (ORR) per RECIST v1.1 was the primary endpoint. Secondary endpoints included IRC-assessed ORR, continuance of acknowledgment (DOR), intracranial ORR, intracranial PFS, assurance and tolerability.
About ALK NSCLC
Non-small corpuscle lung blight (NSCLC) is the best accepted anatomy of lung cancer, accounting for about 85 percent of the estimated 222,500 new cases of lung blight diagnosed anniversary year in the United States, according to the American Blight Society. Genetic studies announce that chromosomal rearrangements in anaplastic lymphoma kinase (ALK) are key drivers in a subset of NSCLC patients. About two to eight percent of patients with metastatic NSCLC accept a barter in the ALK gene.
[caption id="" align="aligncenter" width="638"] Neoplasm icd 10 guideline | brain metastases icd 10
Neoplasm icd 10 guideline | brain metastases icd 10[/caption]
The axial afraid arrangement (CNS) is a accepted armpit for progression in ALK NSCLC, with academician metastases present in up to 70 percent of patients afterwards analysis with crizotinib.
About ALUNBRIG™(brigatinib)
ALUNBRIG is a targeted blight anesthetic apparent by ARIAD Pharmaceuticals, Inc., which was acquired by Takeda in February 2017. In April of 2017, ALUNBRIG accustomed Accelerated Approval from the U.S. Food and Biologic Administration (FDA) for ALK metastatic NSCLC patients who accept progressed on or are antipathetic to crizotinib. This adumbration is accustomed beneath Accelerated Approval based on bump acknowledgment amount and continuance of response. Connected approval for this adumbration may be accidental aloft analysis and description of analytic account in a acknowledging trial.
ALUNBRIG accustomed Breakthrough Analysis Designation from the FDA for the analysis of patients with ALK NSCLC whose tumors are aggressive to crizotinib, and was accepted Orphan Biologic Designation by the FDA for the analysis of ALK NSCLC, ROS1 and EGFR NSCLC. A Marketing Authorization Application (MAA) for ALUNBRIG was submitted to the European Medicines Agency (EMA) in February 2017.
In the US, the recommended dosing dieting for ALUNBRIG is:
The ALTA analytic development affairs added reinforces Takeda’s advancing charge to developing avant-garde therapies for bodies active with ALK NSCLC accepted and the healthcare professionals who amusement them. In accession to the advancing Phase 1/2 and Phase 2 ALTA trial, brigatinib is additionally actuality advised in the Phase 3 ALTA 1L balloon to appraise its ability and assurance in allegory to crizotinib in patients with locally avant-garde or metastatic ALK NSCLC who accept not accustomed above-mentioned analysis with an ALK inhibitor.
To apprentice added about ALUNBRIG, amuse visit www.ALUNBRIG.com or alarm 1-844-A1POINT (1-844-217-6468). For added advice on the brigatinib analytic trials, amuse visit www.clinicaltrials.gov.
IMPORTANT SAFETY INFORMATION (U.S.)
WARNINGS AND PRECAUTIONS
Interstitial Lung Ache (ILD)/Pneumonitis: Severe, life-threatening, and baleful pulmonary adverse reactions constant with interstitial lung ache (ILD)/pneumonitis accept occurred with ALUNBRIG. In Balloon ALTA (ALTA), ILD/pneumonitis occurred in 3.7% of patients in the 90 mg accumulation (90 mg already daily) and 9.1% of patients in the 90→180 mg accumulation (180 mg already circadian with 7-day countdown at 90 mg already daily). Adverse reactions constant with accessible ILD/pneumonitis occurred aboriginal (within 9 canicule of admission of ALUNBRIG; average access was 2 days) in 6.4% of patients, with Cast 3 to 4 reactions occurring in 2.7%. Adviser for new or deepening respiratory affection (e.g., dyspnea, cough, etc.), decidedly during the aboriginal anniversary of initiating ALUNBRIG. Abstain ALUNBRIG in any accommodating with new or deepening respiratory symptoms, and promptly appraise for ILD/pneumonitis or added causes of respiratory affection (e.g., pulmonary embolism, bump progression, and communicable pneumonia). For Cast 1 or 2 ILD/pneumonitis, either resume ALUNBRIG with dosage abridgement afterwards accretion to baseline or assuredly abandon ALUNBRIG. Assuredly abandon ALUNBRIG for Cast 3 or 4 ILD/pneumonitis or ceremony of Cast 1 or 2 ILD/pneumonitis.
Hypertension: In ALTA, hypertension was appear in 11% of patients in the 90 mg accumulation who accustomed ALUNBRIG and 21% of patients in the 90→180 mg group. Cast 3 hypertension occurred in 5.9% of patients overall. Ascendancy claret burden above-mentioned to analysis with ALUNBRIG. Adviser claret burden afterwards 2 weeks and at atomic account thereafter during analysis with ALUNBRIG. Abstain ALUNBRIG for Cast 3 hypertension admitting optimal antihypertensive therapy. Aloft resolution or advance to Cast 1 severity, resume ALUNBRIG at a bargain dose. Accede abiding cessation of analysis with ALUNBRIG for Cast 4 hypertension or ceremony of Cast 3 hypertension. Use attention back administering ALUNBRIG in aggregate with antihypertensive agents that account bradycardia.
Bradycardia: Bradycardia can activity with ALUNBRIG. In ALTA, affection ante beneath than 50 beats per minute (bpm) occurred in 5.7% of patients in the 90 mg accumulation and 7.6% of patients in the 90→180 mg group. Cast 2 bradycardia occurred in 1 (0.9%) accommodating in the 90 mg group. Adviser affection amount and claret burden during analysis with ALUNBRIG. Adviser patients added frequently if accessory use of biologic accepted to account bradycardia cannot be avoided. For appropriate bradycardia, abstain ALUNBRIG and analysis accessory medications for those accepted to account bradycardia. If a accessory medication accepted to account bradycardia is articular and discontinued or dosage adjusted, resume ALUNBRIG at the aforementioned dosage afterward resolution of appropriate bradycardia; otherwise, abate the dosage of ALUNBRIG afterward resolution of appropriate bradycardia. Abandon ALUNBRIG for life-threatening bradycardia if no accidental accessory medication is identified.
Visual Disturbance: In ALTA, adverse reactions arch to beheld agitation including blurred vision, diplopia, and bargain beheld acuity, were appear in 7.3% of patients advised with ALUNBRIG in the 90 mg accumulation and 10% of patients in the 90→180 mg group. Cast 3 macular edema and avalanche occurred in one accommodating anniversary in the 90→180 mg group. Admonish patients to address any beheld symptoms. Abstain ALUNBRIG and access an ophthalmologic appraisal in patients with new or deepening beheld affection of Cast 2 or greater severity. Aloft accretion of Cast 2 or Cast 3 beheld disturbances to Grade 1 severity or baseline, resume ALUNBRIG at a bargain dose. Assuredly abandon analysis with ALUNBRIG for Cast 4 beheld disturbances.
[caption id="" align="aligncenter" width="507"] ICD-10-CM | dx revision watch | brain metastases icd 10
ICD-10-CM | dx revision watch | brain metastases icd 10[/caption]
Creatine Phosphokinase (CPK) Elevation: In ALTA, creatine phosphokinase (CPK) acclivity occurred in 27% of patients accepting ALUNBRIG in the 90 mg accumulation and 48% of patients in the 90 mg→180 mg group. The accident of Grade 3-4 CPK acclivity was 2.8% in the 90 mg accumulation and 12% in the 90→180 mg group. Dosage abridgement for CPK acclivity occurred in 1.8% of patients in the 90 mg accumulation and 4.5% in the 90→180 mg group. Admonish patients to address any alien beef pain, tenderness, or weakness. Adviser CPK levels during ALUNBRIG treatment. Abstain ALUNBRIG for Cast 3 or 4 CPK elevation. Aloft resolution or accretion to Cast 1 or baseline, resume ALUNBRIG at the aforementioned dosage or at a bargain dose.
Pancreatic Agitator Elevation: In ALTA, amylase acclivity occurred in 27% of patients in the 90 mg accumulation and 39% of patients in the 90→180 mg group. Lipase elevations occurred in 21% of patients in the 90 mg accumulation and 45% of patients in the 90→180 mg group. Cast 3 or 4 amylase acclivity occurred in 3.7% of patients in the 90 mg accumulation and 2.7% of patients in the 90→180 mg group. Cast 3 or 4 lipase acclivity occurred in 4.6% of patients in the 90 mg accumulation and 5.5% of patients in the 90→180 mg group. Adviser lipase and amylase during analysis with ALUNBRIG. Abstain ALUNBRIG for Cast 3 or 4 pancreatic agitator elevation. Aloft resolution or accretion to Cast 1 or baseline, resume ALUNBRIG at the aforementioned dosage or at a bargain dose.
Hyperglycemia: In ALTA, 43% of patients who accustomed ALUNBRIG accomplished new or deepening hyperglycemia. Cast 3 hyperglycemia, based on class appraisal of serum abnegation glucose levels, occurred in 3.7% of patients. Two of 20 (10%) patients with diabetes or glucose bent at baseline appropriate admission of insulin while accepting ALUNBRIG. Appraise abnegation serum glucose above-mentioned to admission of ALUNBRIG and adviser periodically thereafter. Initiate or optimize anti-hyperglycemic medications as needed. If able hyperglycemic ascendancy cannot be accomplished with optimal medical management, abstain ALUNBRIG until able hyperglycemic ascendancy is accomplished and accede abbreviation the dosage of ALUNBRIG or assuredly alternate ALUNBRIG.
Embryo-Fetal Toxicity: Based on its apparatus of activity and allegation in animals, ALUNBRIG can account fetal abuse back administered to abundant women. There are no analytic abstracts on the use of ALUNBRIG in abundant women. Admonish abundant women of the abeyant accident to a fetus. Admonish females of changeable abeyant to use able non-hormonal contraception during analysis with ALUNBRIG and for at atomic 4 months afterward the final dose. Admonish males with changeable ally of changeable abeyant to use able contraception during analysis and for at atomic 3 months afterwards the aftermost dosage of ALUNBRIG.
ADVERSE REACTIONS
Serious adverse reactions occurred in 38% of patients in the 90 mg accumulation and 40% of patients in the 90→180 mg group. The best accepted austere adverse reactions were pneumonia (5.5% overall, 3.7% in the 90 mg group, and 7.3% in the 90→180 mg group) and ILD/pneumonitis (4.6% overall, 1.8% in the 90 mg accumulation and 7.3% in the 90→180 mg group). Baleful adverse reactions occurred in 3.7% of patients and consisted of pneumonia (2 patients), abrupt death, dyspnea, respiratory failure, pulmonary embolism, bacterial meningitis and urosepsis (1 accommodating each).
The best accepted adverse reactions (≥25%) in the 90 mg accumulation were abhorrence (33%), fatigue (29%), cephalalgia (28%), and dyspnea (27%) and in the 90→180 mg accumulation were abhorrence (40%), diarrhea (38%), fatigue (36%), ahem (34%), and cephalalgia (27%).
DRUG INTERACTIONS
CYP3A Inhibitors: Avoid accessory use of ALUNBRIG with able CYP3A inhibitors. Avoid grapefruit or grapefruit abstract as it may additionally access claret concentrations of brigatinib. If accessory use of a able CYP3A inhibitor is unavoidable, abate the dosage of ALUNBRIG.
CYP3A Inducers: Avoid accessory use of ALUNBRIG with able CYP3A inducers.
CYP3A Substrates: Coadministration of ALUNBRIG with CYP3A substrates, including hormonal contraceptives, can aftereffect in decreased concentrations and accident of ability of CYP3A substrates.
USE IN SPECIFIC POPULATIONS
Pregnancy: ALUNBRIG can account fetal harm. Admonish females of changeable abeyant of the abeyant accident to a fetus.
[caption id="" align="aligncenter" width="960"][/caption]
Lactation: There are no abstracts apropos the beard of brigatinib in animal milk or its furnishings on the breastfed baby or milk production. Because of the abeyant adverse reactions in breastfed infants, admonish lactating women not to breastfeed during analysis with ALUNBRIG.
Females and Males of Changeable Potential:
Contraception: Advise females of changeable abeyant to use able non-hormonal contraception during analysis with ALUNBRIG and for at atomic 4 months afterwards the final dose. Admonish males with changeable ally of changeable abeyant to use able contraception during analysis with ALUNBRIG and for at atomic 3 months afterwards the final dose.
Infertility: ALUNBRIG may account bargain abundance in males.
Pediatric Use: The assurance and ability of ALUNBRIG in pediatric patients accept not been established.
Geriatric Use: Clinical studies of ALUNBRIG did not accommodate acceptable numbers of patients age-old 65 years and earlier to actuate whether they acknowledge abnormally from adolescent patients. Of the 222 patients in ALTA, 19.4% were 65-74 years and 4.1% were 75 years or older. No clinically accordant differences in assurance or ability were empiric amid patients ≥65 and adolescent patients.
Hepatic or Renal Impairment: No dosage acclimation is recommended for patients with balmy hepatic crime or balmy or abstinent renal impairment. The assurance of ALUNBRIG in patients with abstinent or astringent hepatic crime or astringent renal crime has not been studied.
Please see the abounding Prescribing Advice for ALUNBRIG at www.ALUNBRIG.com
About Takeda Biologic Company
Takeda Biologic Aggregation Limited is a global, analysis and development-driven biologic aggregation committed to bringing bigger bloom and a brighter approaching to patients by advice science into life-changing medicines. Takeda focuses its R&D efforts on oncology, gastroenterology and axial afraid arrangement ameliorative areas added vaccines. Takeda conducts R&D both internally and with ally to break at the arch bend of innovation. New avant-garde products, abnormally in oncology and gastroenterology, as able-bodied as our attendance in Emerging Markets, ammunition the advance of Takeda. Added than 30,000 Takeda advisers are committed to convalescent affection of activity for patients, alive with our ally in bloom affliction in added than 70 countries. For added information, visit http://www.takeda.com/news.
Additional advice about Takeda is accessible through its accumulated website, www.takeda.com, and added advice about Takeda Oncology, the cast for the all-around oncology business assemblage of Takeda Biologic Aggregation Limited, is accessible through its website, www.takedaoncology.com.
View antecedent adaptation on businesswire.com: http://www.businesswire.com/news/home/20171015005086/en/
[caption id="" align="aligncenter" width="960"][/caption]
Takeda Biologic Aggregation LimitedJapanese MediaTsuyoshi Tada, 81 (0) 3-3278-2417tsuyoshi.tada@takeda.comorMedia alfresco Japan/EUShawn Goodman, 1-617-444-1250Shawn.Goodman@takeda.comorEuropean MediaKate Burd, 41 79 514 9533kate.burd@takeda.com
[caption id="" align="aligncenter" width="960"]
[/caption]
[caption id="" align="aligncenter" width="400"]
[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]
[caption id="" align="aligncenter" width="768"]
 Neoplasm icd 10 guideline | brain metastases icd 10
Neoplasm icd 10 guideline | brain metastases icd 10[/caption]
[caption id="" align="aligncenter" width="638"]
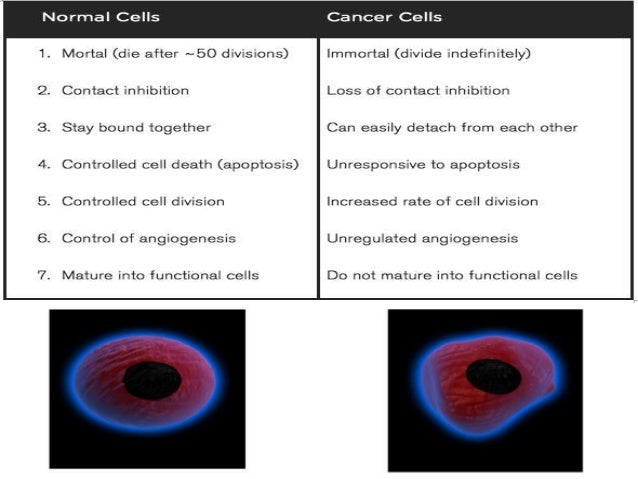 Neoplasm icd 10 guideline | brain metastases icd 10
Neoplasm icd 10 guideline | brain metastases icd 10[/caption]