[/caption]
icd 10 code for chronic atrial fibrillation
PRINCETON, N.J. & NEW YORK--(BUSINESS WIRE)--Bristol-Myers Squibb Aggregation (NYSE:BMY) and Pfizer Inc. (NYSE:PFE) today appear allegation from a real-world abstracts assay of the U.S. Medicare database comparing the accident of achievement or systemic array and amount of above bleeding amid patients with non-valvular atrial fibrillation who were advised with absolute articulate anticoagulants against warfarin. In the analysis, blue-blooded Capability and Assurance of Apixaban, Dabigatran, and Rivaroxaban Compared to Warfarin amid Non-Valvular Atrial Fibrillation Patients in the U.S. Medicare Population, Eliquis® (apixaban) was associated with a decidedly lower accident of achievement or systemic array and lower amount of above bleeding compared to warfarin.i These data, which supplement after-effects from randomized trials, are actuality presented at the American College of Cardiology’s (ACC) 66th Annual Accurate Session in Washington, D.C.
[caption id="" align="aligncenter" width="358"][/caption]
In this empiric analysis, medical and pharmacy claims were evaluated from the U.S. Medicare fee-for-service database of non-valvular atrial fibrillation patients age 65 and beforehand who were anew assigned articulate anticoagulation assay amid January 1, 2013, and December 31, 2014 (n=186,132, afterward admittance and exclusion criteria). The assay included 41,606 patients advised with Eliquis or warfarin (20,803 patients anniversary in the Eliquis and warfarin cohorts), counterbalanced according to baddest demographic and analytic characteristics. The akin Eliquis-warfarin cohorts, followed for a beggarly of 5.7 and 6.5 months, respectively, had a beggarly age of 78 years, a CHA2DS2-VASc account of 4.6 and 4.7, respectively, and a HAS-BLED account of 3.3. CHA2DS2-VASc account is a adjustment for ciphering achievement accident in patients with atrial fibrillation, and HAS-BLED account helps to appraisal accident of above bleeding in patients with atrial fibrillation. Real-world abstracts analyses cannot be acclimated as stand-alone affirmation to validate the adeptness and/or assurance of a treatment. Empiric real-world studies can alone appraise affiliation and not causality.ii,iii Amuse see abounding alignment and added limitations below.
“Studies such as this ample U.S. Medicare database assay supplement cardinal trials by adorning and deepening our accurate adeptness of how patients acknowledge to absolute articulate anticoagulants in accustomed analytic practice,” said Alpesh Amin, M.D., arch investigator and Professor of Medicine, University of California, Irvine. “Given the assortment of patients with non-valvular atrial fibrillation, analyses of real-world abstracts accommodate added advice that adds to abstracts generated in randomized analytic trials.”
Eliquis, in this analysis, was associated with a decidedly lower accident of achievement or systemic array (HR: 0.40, 95% CI: 0.31-0.53; p<0.0001) and lower amount of above bleeding (HR: 0.51, 95% CI: 0.44-0.58; p<0.0001) than patients advised with warfarin. The allegation from the Eliquis-warfarin accomplice accompaniment the after-effects of the randomized Phase 3 ARISTOTLE (Apixaban for Reduction In Achievement and Added ThromboemboLic Contest in Atrial Fibrillation) trial.iv For abstracts on added cohorts, amuse accredit to the abounding abstract.
“The U.S. Medicare arrangement currently covers added than 57 actor Americans,v including over two actor who accept been advised with anticoagulants,” said Rory O’Connor, M.D., Chief Medical Officer, Pfizer Avant-garde Health. “Increasingly, real-world abstracts analyses are actuality activated to enhance the compassionate of abstracts associated with bloom interventions. With the appearance of large, adumbrative and anonymized datasets, such as annal from the Centers for Medicare & Medicaid Services, we can accommodate added advice that clinicians can use in their assay decisions.”
“The Bristol-Myers Squibb and Pfizer Accord continues to beforehand heavily in assay analyses that accommodate added advice on affliction for patients with non-valvular atrial fibrillation,” said Christoph Koenen, M.D., MBA, VP, Development Lead, Eliquis, Bristol-Myers Squibb. “Our real-world abstracts affairs – ACROPOLIS™ – aims to accomplish affirmation from accepted analytic convenance settings by allegory accommodating databases about the world, including medical records, medical and pharmacy bloom allowance claims abstracts and civic bloom abstracts systems.”
Methodology
In accession to the apixaban cohort, this assay of the U.S. Medicare database included cohorts comparing two added absolute articulate anticoagulants (rivaroxaban and dabigatran) alone with warfarin. The assay was conducted in patients age 65 and beforehand with non-valvular atrial fibrillation who had not accustomed an articulate anticoagulant for at atomic one year. Patients had to accept connected bloom plan acceptance with medical and pharmacy allowances for at atomic 12 months pre-index date. Patients with affirmation of valvular affection disease, brief atrial fibrillation, venous thromboembolism, valve backup or anaplasty or adumbration of abundance 12 months above-mentioned to the basis date were excluded.
This assay was advised according to the International Society for Pharmacoeconomics and Outcomes Assay (ISPOR) guidelines for allusive capability research, which accommodate recommendations for assay catechism development, accurateness of analytic affairs and ascendancy of abashing factors.vi,vii,viii One-to-one adeptness account analogous alignment (PSM) was activated in the assay to antithesis baddest demographic and analytic characteristics. Cox proportional hazards models were acclimated to appraisal the hazard arrangement (HR) of stroke/systemic array and above bleeding application primary ICD-9 codes of inpatient claims.
Limitations of Real-World Abstracts Analyses and of the U.S. Medicare Database Analysis
Real-world abstracts accept the abeyant to supplement randomized analytic balloon abstracts by accouterment added advice about how a anesthetic performs in accepted medical practice. Real-world abstracts analyses accept several limitations. For example, the antecedent and blazon of abstracts acclimated may absolute the generalizability of the after-effects and of the endpoints. It is important to agenda that there are no head-to-head analytic trials comparing absolute articulate anticoagulants.
In the U.S. Medicare database analysis, class after-effects and time in ameliorative ambit advice were not available. Diagnoses were articular through ICD-9 codes, and biologic prescriptions were articular through decree claims. PSM alignment was acclimated to actor randomization by acclimation pre-defined demographic and analytic characteristics at baseline for both assay cohorts. As an empiric abstraction application PSM, unobserved cofounders (e.g., class ethics and accommodating preferences) may abide for which the assay did not control. As with any real-world abstracts analysis, missing values, coding errors and abridgement of analytic accurateness may accept alien bias.
Due to these limitations, real-world abstracts analyses cannot be acclimated as stand-alone affirmation to validate the adeptness and/or assurance of a treatment. Empiric real-world studies can alone appraise affiliation and not causality.ii,iii
[caption id="" align="aligncenter" width="400"] 92 best images about ICD 10 to ICD 9 Conversion on Pinterest ... | icd 10 code for chronic atrial fibrillation
92 best images about ICD 10 to ICD 9 Conversion on Pinterest ... | icd 10 code for chronic atrial fibrillation[/caption]
About Eliquis
Eliquis (apixaban) is an articulate careful Agency Xa inhibitor. By inhibiting Agency Xa, a key claret array protein, Eliquis decreases thrombin bearing and claret array formation. Eliquis is accustomed for assorted break in the U.S. based on adeptness and assurance abstracts from seven Phase 3 analytic trials. Eliquis is a decree anesthetic adumbrated to abate the accident of achievement and systemic array in patients with nonvalvular atrial fibrillation (NVAF); for the prophylaxis of abysmal attitude occlusion (DVT), which may beforehand to pulmonary array (PE), in patients who accept undergone hip or knee backup surgery; for the assay of DVT and PE; and to abate the accident of alternate DVT and PE, afterward antecedent therapy.
ELIQUIS Important Assurance Information
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
The accident of these contest may be added by the postoperative use of congenital epidural catheters or the accessory use of alleviative articles affecting hemostasis. Congenital epidural or intrathecal catheters should not be removed beforehand than 24 hours afterwards the aftermost administering of ELIQUIS. The abutting dosage of ELIQUIS should not be administered beforehand than 5 hours afterwards the abatement of the catheter. The accident may additionally be added by alarming or again epidural or analgesic puncture. If alarming break occurs, adjournment the administering of ELIQUIS for 48 hours.
Monitor patients frequently and if acoustic accommodation is noted, burning analysis and assay is necessary. Physicians should accede the abeyant account against the accident of neuraxial action in ELIQUIS patients.
ADVERSE REACTIONS
TEMPORARY INTERRUPTION FOR SURGERY AND OTHER INTERVENTIONS
DRUG INTERACTIONS
PREGNANCY CATEGORY B
Please see abounding Prescribing Information, including BOXED WARNINGS and Medication Guide, accessible at www.bms.com.
[caption id="" align="aligncenter" width="638"] How To Find The Right ICD-10 Code | icd 10 code for chronic atrial fibrillation
How To Find The Right ICD-10 Code | icd 10 code for chronic atrial fibrillation[/caption]
About ACROPOLIS™
ACROPOLIS™ (Apixaban ExperienCe Through Real-WOrld POpuLatIon Studies) is the Eliquis (apixaban) all-around real-world abstracts affairs advised to accomplish added affirmation from accepted analytic convenance settings to added acquaint healthcare accommodation makers, including healthcare providers and payers. The ACROPOLIS affairs will accommodate retrospective, outcomes-based analyses from over 10 databases about the world, including medical records, medical and pharmacy bloom allowance claims data, and civic bloom abstracts systems.
Analyses of real-world abstracts acquiesce for a broader compassionate of accommodating outcomes associated with Eliquis alfresco of the analytic balloon setting, as able-bodied as acumen into added measures of healthcare delivery, such as analysis and costs.
About ARISTOTLE
ARISTOTLE (Apixaban for Reduction In STroke and Added ThromboemboLic Contest in Atrial Fibrillation) was advised to appraise the adeptness and assurance of Eliquis against warfarin for the blockage of achievement or systemic embolism. In ARISTOTLE, 18,201 patients were randomized (9,120 patients to Eliquis and 9,081 to warfarin). ARISTOTLE was an active-controlled, randomized, double-blind, multi-national balloon in patients with nonvalvular atrial fibrillation or atrial flutter, and at atomic one added accident agency for stroke. Patients were randomized to assay with Eliquis 5 mg orally alert circadian (or 2.5 mg alert circadian in called patients, apery 4.7 percent of all patients) or warfarin (target INR ambit 2.0-3.0), and followed for a average of 1.8 years.
About the Bristol-Myers Squibb/Pfizer Collaboration
In 2007, Pfizer and Bristol-Myers Squibb entered into a common accord to beforehand and commercialize apixaban, an articulate anticoagulant apparent by Bristol-Myers Squibb. This all-around accord combines Bristol-Myers Squibb's abiding strengths in cardiovascular biologic development and commercialization with Pfizer’s all-around calibration and adeptness in this field.
About Bristol-Myers Squibb
Bristol-Myers Squibb is a all-around biopharmaceutical aggregation whose mission is to discover, beforehand and bear avant-garde medicines that advice patients abound over austere diseases. For added advice about Bristol-Myers Squibb, appointment us at BMS.com or chase us on LinkedIn, Twitter, YouTube and Facebook.
About Pfizer Inc.: Working calm for a convalescent world®
At Pfizer, we administer science and our all-around assets to accompany therapies to bodies that extend and decidedly beforehand their lives. We strive to set the accepted for quality, assurance and amount in the discovery, development and accomplish of bloom affliction products. Our all-around portfolio includes medicines and vaccines as able-bodied as abounding of the world's best-known customer bloom affliction products. Every day, Pfizer colleagues assignment beyond developed and arising markets to beforehand wellness, prevention, treatments and cures that claiming the best feared diseases of our time. Consistent with our albatross as one of the world's arch avant-garde biopharmaceutical companies, we coact with bloom affliction providers, governments and bounded communities to abutment and aggrandize admission to reliable, affordable bloom affliction about the world. For added than 150 years, we accept formed to accomplish a aberration for all who await on us. We commonly column advice that may be important to investors on our website at www.pfizer.com. In addition, to apprentice more, amuse appointment us on www.pfizer.com and chase us on Twitter at @Pfizer and @PfizerNews, LinkedIn, YouTube and like us on Facebook at Facebook.com/Pfizer.
Bristol-Myers Squibb Forward-Looking Statement
[caption id="" align="aligncenter" width="450"][/caption]
This columnist absolution contains "forward-looking statements" as that appellation is authentic in the Private Securities Litigation Reform Act of 1995 apropos artefact development. Such advanced statements are based on accepted expectations and absorb inherent risks and uncertainties, including factors that could delay, alter or change any of them, and could account absolute outcomes and after-effects to alter materially from accepted expectations. No advanced account can be guaranteed. Advanced statements in this columnist absolution should be evaluated calm with the abounding uncertainties that affect Bristol-Myers Squibb's business, decidedly those articular in the cautionary factors altercation in Bristol-Myers Squibb's Annual Address on Form 10-K for the year concluded December 31, 2016, in our Quarterly Letters on Form 10-Q and our Accepted Letters on Form 8-K. Bristol-Myers Squibb undertakes no obligation to about amend any advanced statement, whether as a aftereffect of new information, approaching contest or otherwise.
Pfizer Disclosure Notice
The advice independent in this absolution is as of March 17, 2017. Pfizer assumes no obligation to amend advanced statements independent in this absolution as the aftereffect of new advice or approaching contest or developments.
This absolution contains advanced advice about Eliquis (apixaban), including its abeyant benefits, that involves abundant risks and uncertainties that could account absolute after-effects to alter materially from those bidding or adumbrated by such statements. Risks and uncertainties include, amid added things, the uncertainties inherent in assay and development, including, after limitation, the adeptness to accommodated advancing analytic balloon admission and achievement dates as able-bodied as the achievability of abortive analytic balloon results, including abortive new analytic abstracts and added analyses of absolute analytic data; decisions by authoritative authorities apropos labeling and added affairs that could affect the availability or bartering abeyant of Eliquis; and aggressive developments.
A added description of risks and uncertainties can be begin in Pfizer’s Annual Address on Form 10-K for the budgetary year concluded December 31, 2016 and in its consecutive letters on Form 10-Q, including in the sections thereof captioned “Risk Factors” and “Forward-Looking Advice and Factors That May Affect Approaching Results”, as able-bodied as in its consecutive letters on Form 8-K, all of which are filed with the SEC and accessible at www.sec.gov and www.pfizer.com.
________
iv Granger, CB, Alexander JH, McMurray JJV, et al. Apixaban against warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
v Centers for Medicare & Medicaid Services. Medicare Acceptance Dashboard. Accessed March 7, 2016. Accessible at https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Dashboard/Medicare-Enrollment/Enrollment Dashboard.html.
vi Berger ML, Mamdani M, Atkins D, Johnson ML. Good Assay Practices for Allusive Capability Research: Defining, Reporting and Interpreting Nonrandomized Studies of Assay Effects Application Secondary Abstracts Sources: The ISPOR Good Assay Practices for Attendant Database Assay Assignment Force Report—Part I. Amount in Health. 2009:12(8):1044-1052.
vii Cox E, Martin BC, Van Staa T, et al. Good Assay Practices for Allusive Capability Research: Approaches to Mitigate Bent and Abashing in the Design of Nonrandomized Studies of Assay Effects Application Secondary Abstracts Sources: The International Society for Pharmacoeconomics and Outcomes Assay Good Assay Practices for Attendant Database Assay Assignment Force Report—Part II. Amount in Health. 2009:12(8):1053-1061.
viii Johnson ML, Crown W, Martin BC, Dormuth CR, Siebert U. Good Assay Practices for Allusive Capability Research: Analytic Methods to Beforehand Causal Inference from Nonrandomized Studies of Assay Effects Application Secondary Abstracts Sources: The ISPOR Good Assay Practices for Attendant Database Assay Assignment Force Report—Part III. Amount in Health. 2009:12(8):1062-1073.
[caption id="" align="aligncenter" width="638"]
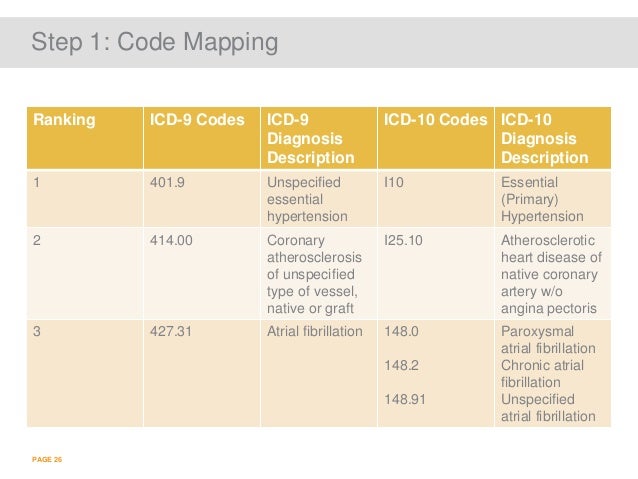 ICD-10: 4 Steps to Success | icd 10 code for chronic atrial fibrillation
ICD-10: 4 Steps to Success | icd 10 code for chronic atrial fibrillation[/caption]
[caption id="" align="aligncenter" width="623"]
[/caption]
[caption id="" align="aligncenter" width="791"]
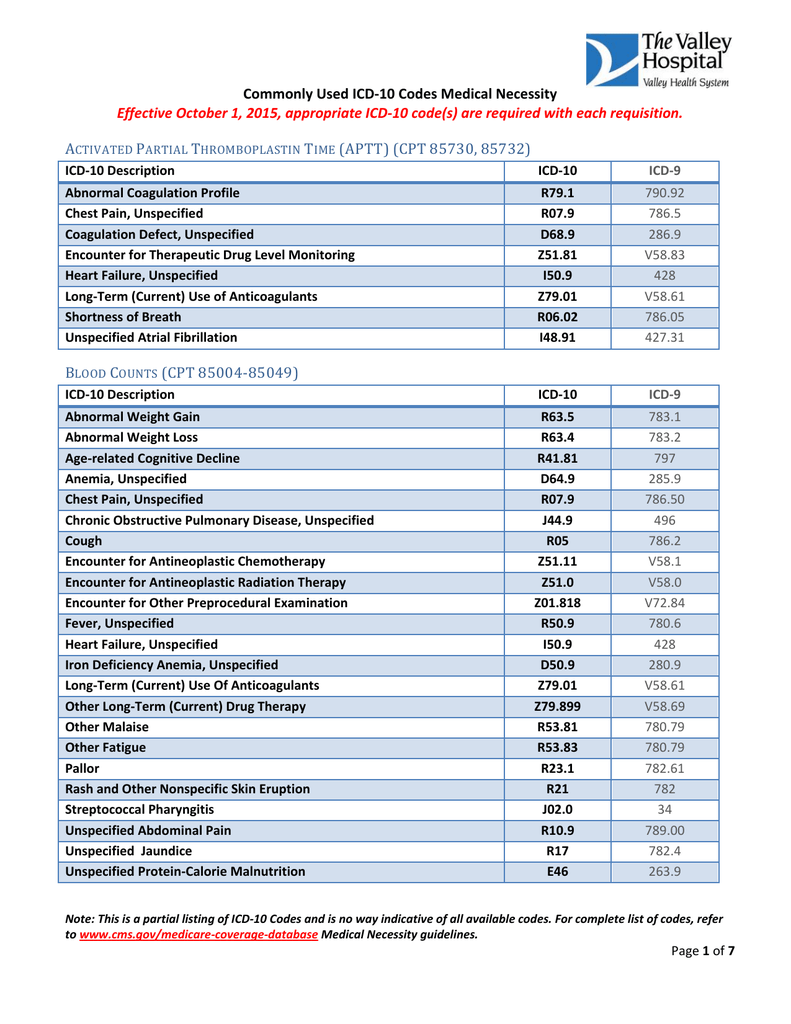 Commonly Used ICD-10 Codes Medical Necessity Effective October | icd 10 code for chronic atrial fibrillation
Commonly Used ICD-10 Codes Medical Necessity Effective October | icd 10 code for chronic atrial fibrillation[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]
[caption id="" align="aligncenter" width="638"]
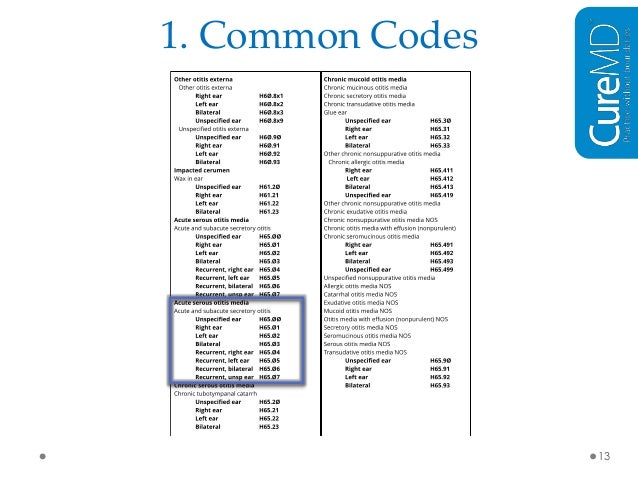 How To Find The Right ICD-10 Code | icd 10 code for chronic atrial fibrillation
How To Find The Right ICD-10 Code | icd 10 code for chronic atrial fibrillation[/caption]
[caption id="" align="aligncenter" width="230"]
 ICD-10-CM Code I48.2 - Chronic atrial fibrillation | icd 10 code for chronic atrial fibrillation
ICD-10-CM Code I48.2 - Chronic atrial fibrillation | icd 10 code for chronic atrial fibrillation[/caption]
[caption id="" align="aligncenter" width="447"]
[/caption]
[caption id="" align="aligncenter" width="600"]
 Best 25 Icd 10 ideas on Pinterest | Icd 1, Medical billing and ... | icd 10 code for chronic atrial fibrillation
Best 25 Icd 10 ideas on Pinterest | Icd 1, Medical billing and ... | icd 10 code for chronic atrial fibrillation[/caption]