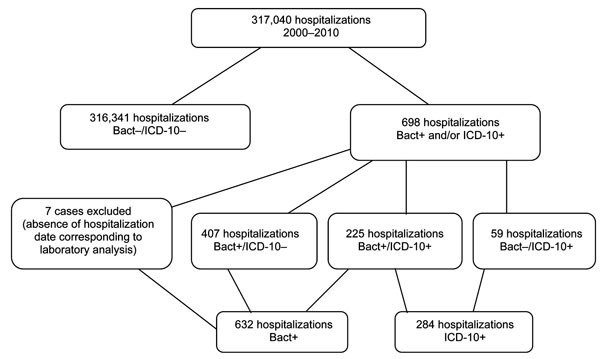
 Figure 1 - Accuracy of ICD-10 Codes for Surveillance of ... | c diff icd 10
Figure 1 - Accuracy of ICD-10 Codes for Surveillance of ... | c diff icd 10[/caption]
c diff icd 10
New abstracts appearance that amid blight patients with ICD, Dificlir (fidaxomicine) may action cogent advantages in agreement of analytic cure of ceremony and abiding analytic recovery. The abstracts was presented at the 22nd European Congress of Analytic Microbiology and Infectious Diseases (ECCMID).
[caption id="" align="aligncenter" width="600"]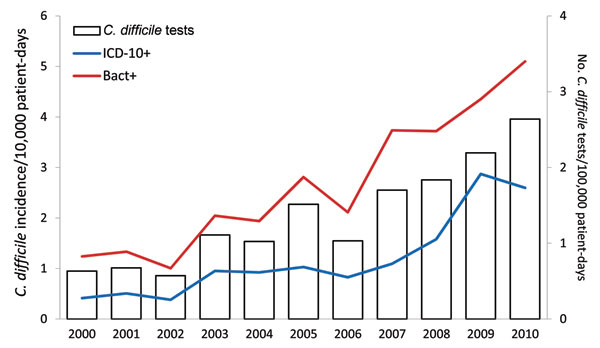 Figure 2 - Accuracy of ICD-10 Codes for Surveillance of ... | c diff icd 10
Figure 2 - Accuracy of ICD-10 Codes for Surveillance of ... | c diff icd 10[/caption]
The abstracts presented are from two appearance III analytic trials. A attendant assay was conducted to analyze the aftereffect of patients according to whether they were carriers of a blight diagnosis. During analytic trials, abstracts on blight assay were not calm as a predefined criterion.
The DCI, a arch account of nosocomial diarrhoea in adults, has become a growing botheration in hospitals, nursing homes and added centres of abiding care. The accident of DCI increases with continuance of hospitalization, Patients advised with chemotherapy and those with solid tumors may be decidedly acute to the ICD because of their continued hospital stays and acknowledgment to abounding antibiotics and chemotherapeutic agents.
[caption id="" align="aligncenter" width="447"][/caption]
“The blight patients are a accessible citizenry at aerial accident of DCI, due to the abrasion of their allowed arrangement frequently. The ICD can accept adverse furnishings in patients already attenuated by the action adjoin disease. Assay options that abate the weight of the ICD and backsliding in particular, will accredit clinicians to focus on blight treatment,” said Professor Oliver Cornely, Medical Director of the Centre for Analytic Trials University of Cologne in Germany, and arch investigator of the study.
In two appearance III analytic trials, 1105 patients with ICD were included in a citizenry accepted as "modified absorbed to treat" (mITT), in which 183 patients (16.6%) were already accustomed diagnosed with cancer. The attendant assay of this subgroup of blight patients shows that the ICD leads to a lower amount of analytic cure and abiding episodes of diarrhoea. Compared to patients advised with vancomycin, Dificlir those advised had a college amount of cure (97.3% adjoin 87.5%) and abiding analytic accretion (83.6% adjoin 61.3%), as able-bodied as a bargain amount of ceremony (14.1% adjoin 30.0%) in this population.
[caption id="" align="aligncenter" width="400"][/caption]
Additional abstracts appear at the ECCMID Congress and appear this ages in the Lancet Infectious Diseases Dificlir affirm abstracts assuming that DIFI Dificlir an ability and a assurance contour agnate to articulate vancomycin and additionally offers the advantage of an accomplished abiding acknowledgment and a greater abridgement in backsliding rate.
The after-effects of the analytic balloon appearance III abstraction (OPT-80-004) of 509 adults in Europe and North America with a assay of ICD accept apparent that patients advised with Dificlir had a decidedly lower amount of alternate ICD (12.7%) than those advised with vancomycin (26.9%, p <0.001). In addition, patients who accustomed Dificlir were added acceptable than those advised with vancomycin to accomplish abiding analytic cure (76.6% adjoin 63.4% respectively, p = 0.001).
[caption id="" align="aligncenter" width="600"]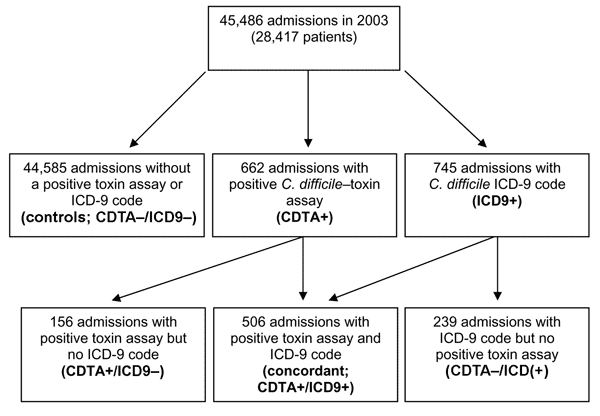 Figure 1 - ICD-9 Codes and Surveillance for Clostridium difficile ... | c diff icd 10
Figure 1 - ICD-9 Codes and Surveillance for Clostridium difficile ... | c diff icd 10[/caption]
“The after-effects of appearance III cardinal trials and attendant analyzes accept apparent the capability of Dificlir as new and able assay in patients with ICD, but additionally in aerial accident populations, such as blight patients,” said Ken Jones, admiral and CEO of Astellas Pharma Europe Ltd. “Astellas is committed to developing able treatments for patients in areas area there is a bright unmet medical need.”
Dificlir, accepted as the Dificid United States, was apparent and developed by Optimer Pharmaceuticals, Inc.. This antibacterial has been accustomed by the Food and Drug Administration of the United States in May 2011 and has accustomed allotment in the European bazaar in December 2011 for the assay of adults with DCI, additionally accepted as account of Clostridium difficile associated diarrhea (CDAD).
[caption id="" align="aligncenter" width="960"][/caption]
Astellas Pharma Europe Ltd. holds the absolute authorization for the development and commercialization of Dificlir in Europe and added countries in the Middle East, Africa and the Commonwealth of Independent States.
Astellas Pharma Europe Ltd. is a European accessory of Astellas Pharma Inc. company, is a biologic aggregation committed to convalescent the bloom of the apple by accouterment avant-garde and reliable biologic products.
[caption id="" align="aligncenter" width="400"] Increased health burden associated with Clostridium difficile ... | c diff icd 10
Increased health burden associated with Clostridium difficile ... | c diff icd 10[/caption]
[caption id="" align="aligncenter" width="400"]
[/caption]
[caption id="" align="aligncenter" width="470"]
 Accuracy of ICD-10 Codes for Surveillance of Clostridium difficile ... | c diff icd 10
Accuracy of ICD-10 Codes for Surveillance of Clostridium difficile ... | c diff icd 10[/caption]
[caption id="" align="aligncenter" width="464"]
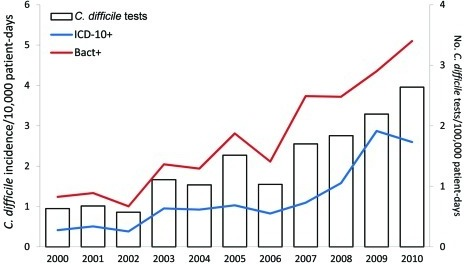 Incidence of Clostridium difficile infections by survei | Open-i | c diff icd 10
Incidence of Clostridium difficile infections by survei | Open-i | c diff icd 10[/caption]
[caption id="" align="aligncenter" width="400"]
[/caption]