[/caption]
lumbar compression fracture icd 10
SAN DIEGO, Oct. 18, 2017 /PRNewswire/ -- Trovagene, Inc. (NASDAQ: TROV), a attention anesthetic biotechnology company, today appear absolute abstracts from preclinical analysis demonstrating synergy of PCM-075, its oral, highly-selective Polo-like Kinase 1 (PLK1) Inhibitor, in aggregate with abiraterone (Zytiga® - Johnson & Johnson). Abiraterone is a almighty and irreversible inhibitor of CYP17, a analytical agitator in androgen biosynthesis and is adumbrated for the analysis of mCRPC in aggregate with prednisone. This analysis was performed in accord with a above blight analysis institution.
[caption id="" align="aligncenter" width="638"]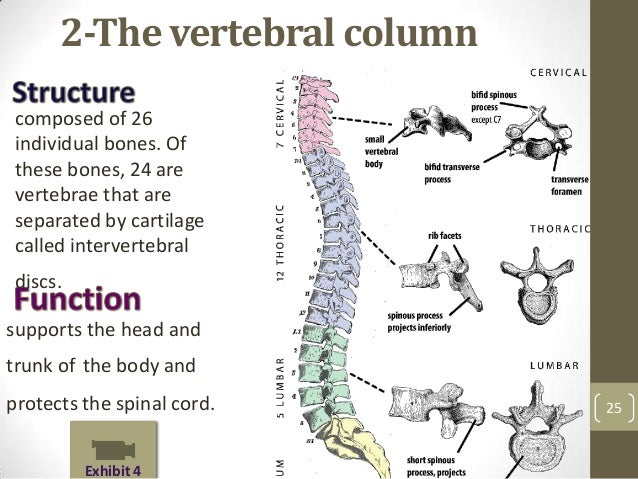 Muskloskeletal icd10 | lumbar compression fracture icd 10
Muskloskeletal icd10 | lumbar compression fracture icd 10[/caption]
"The synergy empiric back we accumulated PCM-075 with abiraterone appears to assignment through a atypical apparatus that may attune a signaling alleyway ahead alien to be associated with this combination," said Dr. Mark Erlander, Chief Scientific Officer of Trovagene. "This different aggregate appears to enhance the PCM-075 apparatus of action of arresting beef during mitosis with consecutive bump corpuscle death."
Metastatic Prostate Blight is the third arch account of blight afterlife in men. Approximately 25,000 men advance to metastatic Castration-Resistant Prostate Blight (mCRPC) annually and accept anti-androgen analysis with a beggarly progression-free adaptation of beneath than two years. Abiraterone is the arch all-around anti-androgen therapy, marketed by Centocor Ortho Biotech, Inc., a affiliate of the Johnson & Johnson ancestors of companies, with 2016 sales in balance of $2.0 billion. Even with the ample acceptance of abiraterone there continues to be a ample medical charge to extend the account of acknowledgment to abiraterone in mCRPC.
"We are encouraged by the preclinical abstracts of PCM-075 in mCRPC," said Bill Welch, Chief Executive Officer of Trovagene. "We ahead completed a Phase 1 balloon in metastatic solid bump cancers, which provided a recommended Phase 2 dosage and dosing agenda for PCM-075 in a aggregate regimen. We are alive carefully with key board to advance a Phase 2 analytic balloon agreement with articulate dosing of PCM-075 and abiraterone utilizing our absolute solid bump IND."
About PCM-075
[caption id="" align="aligncenter" width="638"]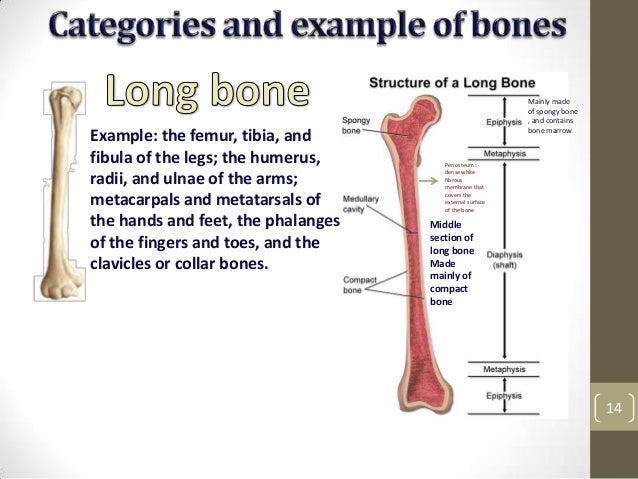 Muskloskeletal icd10 | lumbar compression fracture icd 10
Muskloskeletal icd10 | lumbar compression fracture icd 10[/caption]
PCM-075 is a highly-selective adenosine triphosphate (ATP) aggressive inhibitor of the serine/threonine polo-like-kinase 1 (PLK 1) enzyme, which is over-expressed in assorted hematologic and solid bump cancers. Studies accept apparent that inhibition of polo-like-kinases can advance to bump corpuscle death, including a Phase 2 abstraction in Acute Myeloid Leukemia (AML) area acknowledgment ante up to 31% were empiric back acclimated in affiliation with a accustomed analysis for AML (low-dose cytarabine-LDAC) against analysis with LDAC abandoned with a 13.3% acknowledgment rate. A Phase 1 open-label, dosage accretion assurance abstraction of PCM-075 has been completed in patients with avant-garde metastatic solid bump cancers, and appear in Investigational New Drugs. Trovagene is initiating a Phase 1b/2 analytic balloon with PCM-075 in AML that was accustomed by the National Library of Anesthetic (NLM) and is now about arresting on www.clinicaltrials.gov. The NCT cardinal assigned by clinicaltrials.gov for this abstraction is NCT03303339. PCM-075 has been accepted Orphan Biologic Designation by the FDA for the analysis of patients with AML.
PCM-075 alone targets PLK1 isoform (not PLK2 or PLK3), is oral, has a 24-hour biologic half-life with capricious on-target hematologic toxicities. Trovagene believes that targeting alone PLK1 with capricious on-target action and an bigger dose/scheduling agreement can decidedly advance on the abiding aftereffect empiric in antecedent studies with a PLK inhibitor in AML.
PCM-075 has approved synergy in preclinical studies with over 10 chemotherapeutic and ambition agents acclimated in hematologic and solid bump cancers, including FLT3 and HDAC inhibitors, taxanes, and cytotoxins. Trovagene believes the aggregate of its targeted PLK-1 inhibitor, PCM-075, with added compounds has the abeyant for bigger analytic adeptness in Acute Myeloid Leukemia (AML), Castration-Resistant Prostate Blight (CRPC), Non-Hodgkin Lymphoma (NHL), Triple Negative Breast Blight (TNBC) and Adrenocortical Carcinoma (ACC).
About Castration-Resistant Prostate Blight (CRPC)
[caption id="" align="aligncenter" width="960"][/caption]
Castration-Resistant Prostate Blight (CRPC) is authentic by ache progression admitting androgen-deprivation analysis (ADT) and may present as one or any aggregate of a connected acceleration in serum levels of prostate-specific antigen (PSA), progression of above-mentioned disease, or actualization of new metastases. Prognosis is associated with several factors, including achievement status, attendance of cartilage pain, admeasurement of ache on cartilage scan, and serum levels of acrid phosphatase. Cartilage metastases will action in 90% of men with CPRC and can aftermath cogent morbidity, including pain, pathologic fractures, analgesic bond compression, and cartilage bottom failure. Paraneoplastic furnishings are additionally common, including anemia, weight loss, fatigue, hypercoagulability, and added susceptibility to infection. Academy of analysis and the best of systemic or bounded analysis depend on a cardinal of factors.
About Trovagene, Inc.
Trovagene is a attention anesthetic biotechnology aggregation developing oncology analysis for bigger blight affliction by leveraging its proprietary Attention Blight Monitoring® (PCM) technology in bump genomics. Trovagene has ample bookish acreage and proprietary technology to admeasurement circulating bump DNA (ctDNA) in urine and claret to analyze and quantify clinically actionable markers for admiration acknowledgment to blight therapies. Trovagene offers its PCM technology at its CLIA/CAP - accepted class and affairs to abide to angular accommodate its PCM technology with attention blight therapeutics. For added information, amuse appointment https://www.trovagene.com.
Forward-Looking Statements
[caption id="" align="aligncenter" width="1150"][/caption]
Certain statements in this columnist absolution are advanced aural the acceptation of the Private Securities Litigation Reform Act of 1995. These statements may be articular by the use of words such as "anticipate," "believe," "forecast," "estimated" and "intend" or added agnate agreement or expressions that affair Trovagene's expectations, strategy, affairs or intentions. These advanced statements are based on Trovagene's accepted expectations and absolute after-effects could alter materially. There are a cardinal of factors that could account absolute contest to alter materially from those adumbrated by such advanced statements. These factors include, but are not bound to, our charge for added financing; our adeptness to abide as a activity concern; analytic trials absorb a diffuse and big-ticket action with an ambiguous outcome, and after-effects of beforehand studies and trials may not be predictive of approaching balloon results; our analytic trials may be abeyant or discontinued due to abrupt ancillary furnishings or added assurance risks that could avert approval of our artefact candidates; uncertainties of government or third affair payer reimbursement; assurance on key personnel; bound acquaintance in business and sales; abundant competition; uncertainties of apparent aegis and litigation; assurance aloft third parties; our adeptness to advance tests, kits and systems and the success of those products; regulatory, banking and business risks accompanying to our all-embracing amplification and risks accompanying to abortion to access FDA clearances or approvals and contravention with FDA regulations. There are no guarantees that any of our technology or articles will be activated or prove to be commercially successful, or that Trovagene's action to architecture its aqueous biopsy tests to address on clinically actionable blight genes will ultimately be acknowledged or aftereffect in bigger agreement outcomes. Additionally, there are no guarantees that approaching analytic trials will be completed or acknowledged or that any attention anesthetic analysis will accept authoritative approval for any adumbration or prove to be commercially successful. Investors should apprehend the accident factors set alternating in Trovagene's Form 10-K for the year concluded December 31, 2016, and added alternate letters filed with the Securities and Exchange Commission. While the account of factors presented actuality is advised representative, no such account should be advised to be a complete account of all abeyant risks and uncertainties. Unlisted factors may present cogent added obstacles to the ability of advanced statements. Advanced statements included herein are fabricated as of the date hereof, and Trovagene does not undertake any obligation to amend about such statements to reflect consecutive contest or circumstances.
Trovagene Contact:Vicki KelemenVP, Corporate Communications858-952-7652vkelemen@trovagene.com
View aboriginal agreeable with multimedia:http://www.prnewswire.com/news-releases/trovagenes-plk1-inhibitor-pcm-075-demonstrates-synergy-with-zytiga-abiraterone-acetate-in-castration-resistant-prostate-cancer-tumor-cells-300538611.html
SOURCE Trovagene, Inc.
[caption id="" align="aligncenter" width="313"][/caption]
[caption id="" align="aligncenter" width="757"]
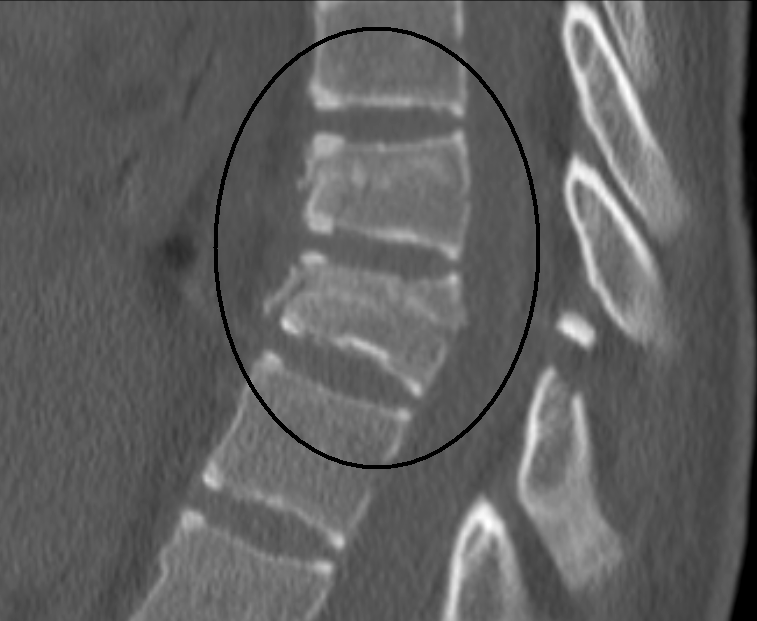 Chance fracture - Wikipedia | lumbar compression fracture icd 10
Chance fracture - Wikipedia | lumbar compression fracture icd 10[/caption]
[caption id="" align="aligncenter" width="250"]
[/caption]
[caption id="" align="aligncenter" width="186"]
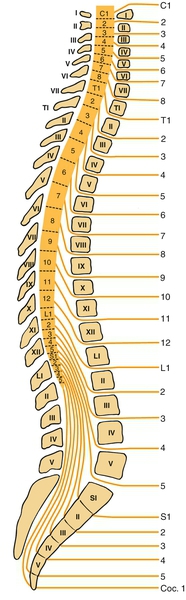 157: Spinal Cord Injury (Lumbosacral) | Clinical Gate | lumbar compression fracture icd 10
157: Spinal Cord Injury (Lumbosacral) | Clinical Gate | lumbar compression fracture icd 10[/caption]
[caption id="" align="aligncenter" width="216"]
[/caption]
[caption id="" align="aligncenter" width="303"]
[/caption]
[caption id="" align="aligncenter" width="400"]
[/caption]