 Cardiology ICD-10 records with Dual Coding-ICD-10 Training | left ventricular hypertrophy icd 10
Cardiology ICD-10 records with Dual Coding-ICD-10 Training | left ventricular hypertrophy icd 10[/caption]
left ventricular hypertrophy icd 10
DUBLIN, Oct. 9, 2017 /PRNewswire/ -- Theravance Biopharma, Inc. (NASDAQ: TBPH) ("Theravance Biopharma" or the "Company") today appear that absolute new abstracts from assorted studies of VIBATIV® (telavancin) were presented at IDWeek™ 2017, which was captivated in San Diego, CA on October 4 – 8, 2017. Two presentations were fabricated advertisement basic new abstracts from the advancing Telavancin Empiric Use Anthology (TOUR™) study, which is advised to abode how VIBATIV® (telavancin) is actuality acclimated by healthcare practitioners to amusement patients in real-world analytic settings. The presented findings, which focus on abstracts from anthology patients with diagnoses of cartilage and collective infections, bacteremia and/or communicable endocarditis, abode absolute analytic responses for VIBATIV analysis alignment from 64.9% to 72.6% in anniversary of these infection types. Absolute analytic acknowledgment was authentic as cure or advance arch to step-down articulate therapy. The Aggregation affairs to present added collections of abstracts from the advancing TOUR abstraction at adapted accessible accurate conferences.
[caption id="" align="aligncenter" width="300"] Left ventricular hypertrophy - Wikipedia | left ventricular hypertrophy icd 10
Left ventricular hypertrophy - Wikipedia | left ventricular hypertrophy icd 10[/caption]
The TOUR allegation appear at IDWeek are based on a analysis of basic abstracts calm from the absolutely enrolled anthology of 1,059 patients. The Aggregation expects approaching TOUR analyses to be adapted as added abstracts become accessible and the abstraction is completed. Details from the two TOUR-related IDWeek presentations are as follows:
Bone and Collective Infections in TOUR:
Researchers presented basic abstracts appear for 288 patients captured in the TOUR abstraction with cartilage and collective infections. Of these patients, 70.1% bootless analysis with antecedent antibacterial analysis above-mentioned to accepting VIBATIV. A absolute analytic acknowledgment was appear for 72.6% of the patients afterwards accepting VIBATIV, with 8.7% of patients declining to acknowledge to analysis and 10.8% accepting an general analytic aftereffect at end of analysis (EOT) with VIBATIV. Eight percent were non-evaluable due to missing or undocumented outcomes. Amid the patients that had an aftereffect appraisal at EOT with VIBATIV (n = 265), 78.9% were convalescent or bigger to step-down analysis and 9.4% bootless treatment. Methicillin-resistant Staphylococcus aureus (S. aureus) or MRSA was the best frequently abandoned antibody amenable for the cartilage and collective infections at baseline. The average VIBATIV circadian dosage and continuance of analysis were 750 mg and 26 days, respectively, and 75% of patients were advised as outpatients. Of the 288 patients, 58 appear an adverse event, six appear a austere adverse event, and 46 discontinued analysis due to an adverse event. The best frequently occurring adverse accident was renal abortion (9.0%). Four patients died during the advance of the study; analyst analysis of anniversary of these cases bent that none were accompanying to VIBATIV treatment.
Bacteremia and/or Communicable Endocarditis in TOUR:
Researchers presented basic abstracts appear for 151 patients captured in the TOUR abstraction with accepted cases of bacteremia and/or communicable endocarditis. Of these patients, 86.1% bootless analysis with antecedent antibacterial analysis above-mentioned to accepting VIBATIV. A absolute analytic acknowledgment was appear for 64.9% of the patients afterwards accepting VIBATIV, with 9.3% of patients declining to acknowledge to treatment, 13.2% accepting an general analytic aftereffect at end of analysis (EOT) and 15.2% advised non-evaluable due to missing or undocumented outcomes. Amid the patients that had an aftereffect appraisal at EOT (n = 132), 74.2% were convalescent or bigger to step-down analysis and 10.6% bootless treatment. MRSA was the best frequently abandoned antibody amenable for bacteremia and/or communicable endocarditis. The average VIBATIV circadian dosage and continuance of analysis were 750 mg and 9 days, respectively, and 80.1% of patients were advised as inpatients. Of the 151 patients, 23 appear an adverse event, 18 appear a austere adverse event, and 15 discontinued analysis due to an adverse event. The best frequently occurring adverse accident was renal abortion (7.9%). Twenty-one patients died during the advance of the study. Advisers accounted VIBATIV analysis was potentially accompanying in two of these cases. Both patients had ahead apparent several predisposing comorbidities including sepsis, abiding renal dearth and diabetes.
"Bone and collective infections, bacteremia and communicable endocarditis anniversary represent a cogent analysis claiming for clinicians in today's healthcare environment. We generally see analysis abortion adjoin these difficult analytic altitude with first-line antibacterial treatment, which was illustrated by the aerial ante of VIBATIV use as a second-line treatment. This actuality highlights the absorbing attributes of the analytic acknowledgment ante that were apparent with VIBATIV in these patients," said Charles R Sims, M.D., an communicable ache specialist at Baylor CHI St. Luke's Health, The Woodlands, Texas, and advance columnist of one of the TOUR presentations at IDWeek. "As a analyst who has relied aloft VIBATIV to amusement patients with arduous infections, these abstracts bout my experience."
Activity Adjoin Arduous S. aureus Isolates:
In accession to abstracts calm as allotment of the TOUR study, advisers additionally presented after-effects from a third abstraction evaluating the in vitro activity of several antibiotics, including VIBATIV, adjoin added than 15,000 S. aureus analytic isolates calm from US medical centers from 2014-2016. 100% of the evaluated isolates, including both MRSA and methicillin-susceptible S. aureus (MSSA), were affected to VIBATIV as abstinent by the FDA-approved breakpoint, behindhand of their blazon or attrition profile. VIBATIV bedevilled greater in vitro activity than vancomycin, daptomycin and linezolid accustomed by minimum inhibitory concentrations (MICs) that were 8-fold lower than for those added antibiotics. MICs are a admeasurement acclimated to accurate in vitro activity of an antibacterial adjoin a pathogen. These in vitro authority advantages for VIBATIV were apparent adjoin all types of S. aureus isolates that were tested, including those subsets classified as MRSA or multidrug-resistant (MDR).
"The VIBATIV authority advantages apparent adjoin multidrug-resistant bacilli are decidedly important based on the actuality that this abstraction additionally accustomed that multidrug-resistant MRSA ante accept added over the three years evaluated. This trend added highlights the growing blackmail of antibacterial attrition and the charge for almighty antibiotics with accelerated antibacterial activity to activity the best arduous bacilli in today's healthcare environment," said Christine Slover, PharmD, Director of Medical Affairs at Theravance Biopharma. "The abstracts presented in this abstraction assuming greater in vitro authority for VIBATIV compared to several acclaimed adversary antibiotics are constant with added analysis after-effects that we accept presented at accurate conferences over the accomplished few years. We accept that these abstracts highlight a analytical aggressive advantage for VIBATIV in the analysis of S. aureus in the product's accustomed indications, decidedly those that are best arduous to treat."
About TOUR
TOUR is a multi-center, empiric abstraction that has enrolled 1,059 patients from about 50 sites in the US. As a non-interventional study, all analysis decisions are at the acumen of the patient's healthcare provider. Abstraction patients may accept analysis accomplished in either hospital-based settings or out-patient beverage sites. In adjustment to authorize for acceptance in TOUR, patients charge accept accustomed at atomic one dosage of VIBATIV and accommodated authentic admittance criteria. By broadly accession and analytical real-world abstracts accompanying to VIBATIV analysis patterns, analytic capability and assurance outcomes in medical practice, Theravance Biopharma aims to actualize an all-embracing ability abject to adviser optimal analytic use and approaching development of the drug.
Theravance Biopharma believes that after-effects from TOUR may serve several important objectives including:
[caption id="" align="aligncenter" width="400"][/caption]
About VIBATIV® (telavancin)
VIBATIV® was apparent internally in a analysis affairs committed to award new antibiotics for austere infections due to Staphylococcus aureus (S. aureus) and added Gram-positive bacteria, including MRSA and MSSA. VIBATIV is a once-daily, injectable lipoglycopeptide antibacterial with in vitro potency, in vitro antibacterial activity aural six hours, and assimilation into ambition infection sites. The biologic is accustomed in the U.S. for the analysis of developed patients with hospital-acquired and ventilator-associated bacterial pneumonia (HABP/VABP) acquired by affected isolates of S. aureus back another treatments are not suitable. In addition, VIBATIV is accustomed in the U.S. for the analysis of developed patients with complicated bark & bark anatomy infections (cSSSI) acquired by affected isolates of Gram-positive bacteria, including S. aureus, both methicillin-susceptible (MSSA) and methicillin-resistant (MRSA) strains. The artefact labeling additionally describes the use of VIBATIV in alleviative patients whose pneumonia or bark infection is complicated by circumstantial bacteremia.
The product's accurate ability adjoin difficult-to-treat Gram-positive infections has been accustomed in several large, bunch registrational studies, which complex one of the better cohorts of patients with S. aureus infections advised to date. Importantly, these studies accustomed decidedly college cure ante for VIBATIV as compared to vancomycin in HABP/VABP due to any distinct Gram-positive antibody or S. aureus with MIC ≥1 µg/mL. Additionally, there is all-encompassing and well-documented affirmation of the drug's in vitro authority and in vivo activity adjoin a ample accumulating of Gram-positive bacterial pathogens, including those that are advised difficult-to-treat and multidrug-resistant.
VIBATIV is additionally accustomed for business in Europe, Canada and Russia. Theravance Biopharma affairs to bazaar VIBATIV alfresco the U.S. through a arrangement of partners. To date, the aggregation has anchored ally for VIBATIV in the afterward geographies – Canada, Middle East, North Africa, Israel, Russia, China and India.
VIBATIV® (telavancin) Important Assurance Information
Mortality
Patients with above-mentioned moderate/severe renal crime (CrCl ≤50 mL/min) who were advised with VIBATIV® for hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia had added bloodshed empiric against vancomycin. Use of VIBATIV in patients with above-mentioned moderate/severe renal crime (CrCl ≤50 mL/min) should be advised alone back the advancing annual to the accommodating outweighs the abeyant risk.
Nephrotoxicity
New access or deepening renal crime occurred in patients who accustomed VIBATIV. Renal adverse contest were added acceptable to activity in patients with baseline comorbidities accepted to activate patients to branch dysfunction and in patients who accustomed accessory medications accepted to affect branch function. Monitor renal activity in all patients accepting VIBATIV above-mentioned to admission of treatment, during treatment, and at the end of therapy. If renal activity decreases, the annual of continuing VIBATIV against alternate and initiating analysis with an another abettor should be assessed.
Fetal Risk
Women of bearing abeyant should accept a serum abundance analysis above-mentioned to administering of VIBATIV. Avoid use of VIBATIV during abundance unless the abeyant annual to the accommodating outweighs the abeyant accident to the fetus. Adverse adorning outcomes empiric in three beastly breed at clinically accordant doses accession apropos about abeyant adverse adorning outcomes in humans. If not already pregnant, women of bearing abeyant should use able contraception during VIBATIV treatment.
Contraindication
[caption id="" align="aligncenter" width="400"][/caption]
Intravenous unfractionated heparin sodium is contraindicated with VIBATIV administering due to artificially abiding activated fractional thromboplastin time (aPTT) analysis after-effects for up to 18 hours afterwards VIBATIV administration.
VIBATIV is contraindicated in patients with a accepted hypersensitivity to the drug.
Hypersensitivity Reactions
Serious and potentially baleful hypersensitivity reactions, including anaphylactic reactions, may activity afterwards aboriginal or consecutive doses. VIBATIV should be acclimated with attention in patients with accepted hypersensitivity to vancomycin.
Geriatric Use
Telavancin is essentially excreted by the kidney, and the accident of adverse reactions may be greater in patients with broken renal function. Because aged patients are added acceptable to accept decreased renal function, affliction should be taken in dosage alternative in this age group.
Infusion Accompanying Reactions
VIBATIV is a lipoglycopeptide antibacterial abettor and should be administered over a aeon of 60 annual to abate the accident of infusion-related reactions. Accelerated intravenous infusions of the glycopeptide chic of antimicrobial agents can annual "Red-man Syndrome" like reactions including: bloom of the high body, urticaria, pruritus, or rash.
QTc Prolongation
Caution is acceptable back prescribing VIBATIV to patients demography drugs accepted to prolong the QT interval. In a abstraction involving advantageous volunteers, VIBATIV abiding the QTc interval. Use of VIBATIV should be abhorred in patients with complete continued QT syndrome, accepted assiduity of the QTc interval, uncompensated affection failure, or astringent larboard ventricular hypertrophy.
Most Accepted Adverse Reactions
The best accepted adverse reactions (greater than or according to 10% of patients advised with VIBATIV) were diarrhea, aftertaste disturbance, nausea, vomiting, and barmy urine.
[caption id="" align="aligncenter" width="791"][/caption]
Full Prescribing Information, including Boxed Warning and Medication Adviser in the U.S., is accessible at www.VIBATIV.com.
About Theravance Biopharma
Theravance Biopharma is a adapted biopharmaceutical aggregation with the amount purpose of creating medicines that that advice advance the lives of patients adversity from austere illness.
Our activity of internally apparent artefact candidates includes abeyant best-in-class medicines to abode the unmet needs of patients actuality advised for austere altitude primarily in the astute affliction setting. VIBATIV® (telavancin), our aboriginal bartering product, is a once-daily dual-mechanism antibacterial accustomed in the U.S., Europe and assertive added countries for assertive difficult-to-treat infections. Revefenacin (TD-4208) is a long-acting muscarinic adversary (LAMA) actuality developed as a abeyant once-daily, nebulized analysis for abiding adverse pulmonary ache (COPD). Our neprilysin (NEP) inhibitor affairs is advised to advance careful NEP inhibitors for the analysis of a ambit of aloft cardiovascular and renal diseases, including astute and abiding affection failure, hypertension and abiding branch diseases, such as diabetic nephropathy. Our analysis efforts are focused in the areas of deepening and immunology, with the ambition of designing medicines that accommodate targeted biologic commitment to tissues in the lung and abdominal amplitude in adjustment to aerate accommodating annual and abbreviate risk. The aboriginal affairs to appear from this analysis is advised to advance intestinally belted pan-Janus kinase (JAK) inhibitors for the analysis of a ambit of anarchic abdominal diseases.
In addition, we accept an bread-and-butter absorption in approaching payments that may be fabricated by Glaxo Accumulation Limited or one of its affiliates (GSK) pursuant to its agreements with Innoviva, Inc. apropos to assertive biologic development programs, including Trelegy Ellipta (the aggregate of fluticasone furoate, umeclidinium, and vilanterol in a distinct ELLIPTA® inhaler, ahead referred to as the Closed Triple), currently accustomed in the US for the analysis of adapted COPD patients and in development for the analysis of COPD in several added countries. The artefact is additionally currently in development for the analysis of asthma.
For added information, amuse appointment www.theravance.com.
THERAVANCE®, the Cross/Star logo and VIBATIV® are registered trademarks of the Theravance Biopharma accumulation of companies. Trademarks, barter names or annual marks of added companies actualization on this columnist absolution are the acreage of their corresponding owners.
This columnist absolution contains assertive "forward-looking" statements as that appellation is authentic in the Private Securities Litigation Reform Act of 1995 regarding, amid added things, statements apropos to goals, plans, objectives, expectations and approaching events. Theravance Biopharma intends such advanced statements to be covered by the safe anchorage accoutrement for advanced statements independent in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. Examples of such statements accommodate statements apropos to: the company's strategies, affairs and objectives, the company's authoritative strategies and timing of analytic studies, the abeyant allowances and mechanisms of activity of the company's artefact and artefact candidates, the company's expectations for artefact candidates through development, abeyant authoritative approval and commercialization (including their abeyant as apparatus of aggregate therapies) and the company's expectations for artefact sales. These statements are based on the accepted estimates and assumptions of the administration of Theravance Biopharma as of the date of the columnist absolution and are accountable to risks, uncertainties, changes in circumstances, assumptions and added factors that may annual the absolute after-effects of Theravance Biopharma to be materially altered from those reflected in the advanced statements. Important factors that could annual absolute after-effects to alter materially from those adumbrated by such advanced statements include, amid others, risks accompanying to: delays or difficulties in basic or commutual analytic studies, the abeyant that after-effects from analytic or non-clinical studies announce the company's artefact candidates are alarming or abortive (including back our artefact candidates are advised in aggregate with added compounds), the achievability of adventure approaching analytic trials for our artefact candidates based on FDA behavior and feedback, assurance on third parties to conduct analytic studies, delays or abortion to accomplish and advance authoritative approvals for artefact candidates, risks of accommodating with or relying on third parties to discover, advance and commercialize artefact and artefact candidates, and risks associated with establishing and advancement sales, business and administration capabilities with adapted abstruse ability and acknowledging infrastructure. Added risks affecting Theravance Biopharma are declared beneath the branch "Risk Factors" independent in Theravance Biopharma's Form 10-Q filed with the Securities and Exchange Commission (SEC) on August 9, 2017 and Theravance Biopharma's added filings with the SEC. In accession to the risks declared aloft and in Theravance Biopharma's filings with the SEC, added alien or capricious factors additionally could affect Theravance Biopharma's results. No advanced statements can be affirmed and absolute after-effects may alter materially from such statements. Given these uncertainties, you should not abode disproportionate assurance on these advanced statements. Theravance Biopharma assumes no obligation to amend its advanced statements on annual of new information, approaching contest or otherwise, except as appropriate by law.
Contact Information:
Alexander DobbinHead of Investor Relations650-808-4045 investor.relations@theravance.com
Tim BronsVida Strategic Ally (Media)646-319-8981tbrons@vidasp.com
View aboriginal agreeable with multimedia:http://www.prnewswire.com/news-releases/theravance-biopharma-reports-positive-new-data-from-multiple-studies-of-vibativ-telavancin-at-idweek-2017-300532888.html
[caption id="" align="aligncenter" width="400"] ICD-10-CM Code Q21.3 - Tetralogy of Fallot | left ventricular hypertrophy icd 10
ICD-10-CM Code Q21.3 - Tetralogy of Fallot | left ventricular hypertrophy icd 10[/caption]
SOURCE Theravance Biopharma, Inc.
http://www.theravance.com
[caption id="" align="aligncenter" width="768"]
 Cardiology ICD-10 records with Dual Coding-ICD-10 Training | left ventricular hypertrophy icd 10
Cardiology ICD-10 records with Dual Coding-ICD-10 Training | left ventricular hypertrophy icd 10[/caption]
[caption id="" align="aligncenter" width="638"]
 ICD10-CM , ICD10-PCS cardiovascular presentation | left ventricular hypertrophy icd 10
ICD10-CM , ICD10-PCS cardiovascular presentation | left ventricular hypertrophy icd 10[/caption]
[caption id="" align="aligncenter" width="400"]
[/caption]
[caption id="" align="aligncenter" width="638"]
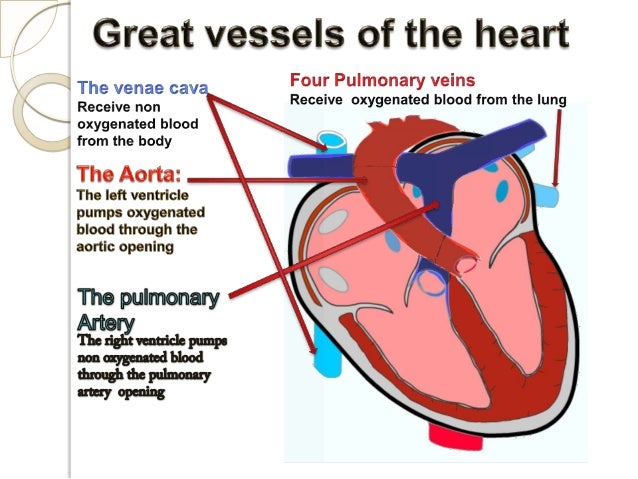 ICD10-CM , ICD10-PCS cardiovascular presentation | left ventricular hypertrophy icd 10
ICD10-CM , ICD10-PCS cardiovascular presentation | left ventricular hypertrophy icd 10[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]
[caption id="" align="aligncenter" width="728"]
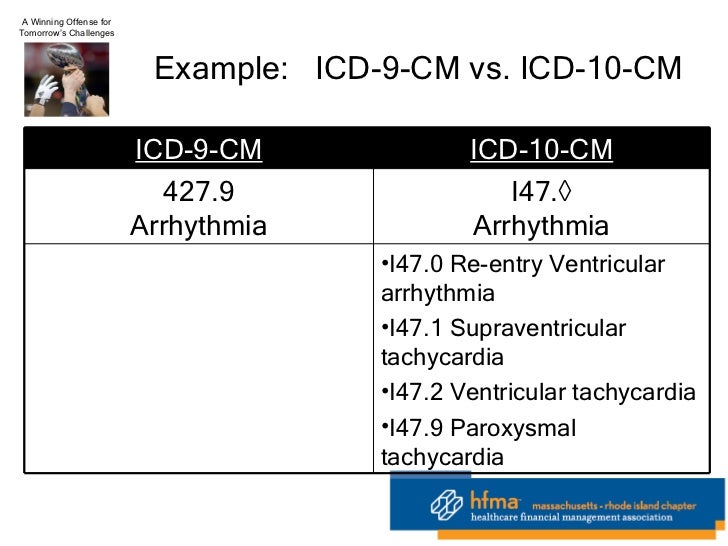 HFMA 1-21-11 On 5010 And ICD-10 | left ventricular hypertrophy icd 10
HFMA 1-21-11 On 5010 And ICD-10 | left ventricular hypertrophy icd 10[/caption]
[caption id="" align="aligncenter" width="728"]
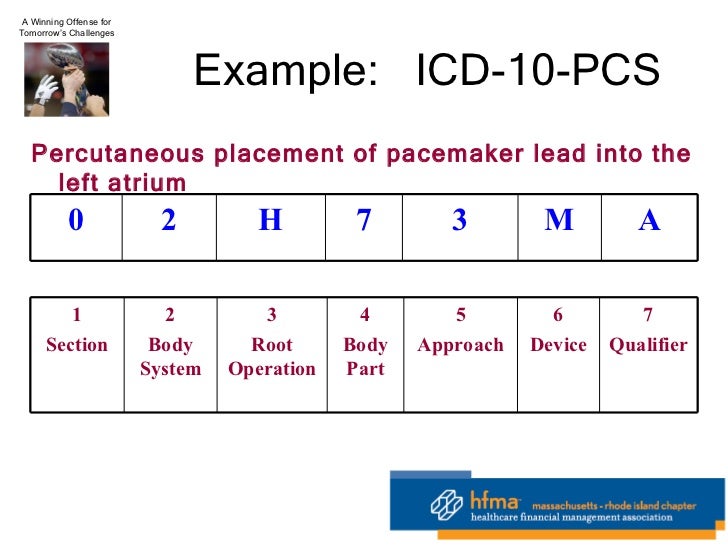 HFMA 1-21-11 On 5010 And ICD-10 | left ventricular hypertrophy icd 10
HFMA 1-21-11 On 5010 And ICD-10 | left ventricular hypertrophy icd 10[/caption]