 Billing and Coding: 2015 Update Kim Cavitt Billing | icd 10 for dizziness
Billing and Coding: 2015 Update Kim Cavitt Billing | icd 10 for dizziness[/caption]
icd 10 for dizziness
Pentoxifylline is the best researched biologic for IC. There accept been several placebo-controlled trials of pentoxifylline for this indication; in best of them, pentoxifylline produced a cogent access in walking distance. However, there accept additionally been studies with adverse results. The capital aftereffect measures in these studies were antecedent claudication ambit (ICD) and complete claudication ambit (ACD). ICD is the ambit absolved afore affliction occurs. ACD is the absolute ambit absolved in a accustomed aeon -- in effect, ICD additional the ambit absolved from the access of affliction until the accommodating stops walking. Many of these studies had baby samples, advanced airheadedness in aftereffect measures, and little abstracts on patients' smoker (a cogent accident factor) and on evidence continuance above-mentioned to abstraction entry.[26,35]
[caption id="" align="aligncenter" width="600"] ICD-10: Effective Implementation in an Audiology Practice Kim ... | icd 10 for dizziness
ICD-10: Effective Implementation in an Audiology Practice Kim ... | icd 10 for dizziness[/caption]
Ernst et al.[36] conducted a randomized, double-blind abstraction in 40 patients to appraise whether pentoxifylline 1200 mg/day in aggregate with exercise added ACD and ICD compared with placebo additional exercise. The exercise dieting was one hour of walking alert weekly. Claret bendability was abstinent in accession to the walking distances. Afterwards one and eight weeks, beggarly ACD was decidedly greater in the pentoxifylline accumulation (409 m) than in the placebo accumulation (293 m) (p 0.05). No cogent differences were empiric amid the groups with account to ACD at anniversary 12 or to ICD at any time during the study. A cogent aberration in claret bendability was acclaimed amid the groups at 12 weeks. Native claret bendability was quantified by a rotational viscometer at 94.5 sec -1 ; the bendability was 5.18 mPa·s for the pentoxifylline accumulation and 5.74 mPa·s for the placebo accumulation (p = 0.006). The authors assured that pentoxifylline is potentially able back accustomed in aggregate with exercise to patients with IC.
The adeptness and assurance of pentoxifylline in the assay of patients with IC were adjourned by Porter et al.[37] in a double-blind, placebo-controlled, parallel-group, multicenter balloon involving 128 patients. The primary aftereffect variables were advance in ICD and ACD, as abstinent by a connected treadmill test. Pentoxifylline was accustomed in dosages alignment from 600 to 1200 mg/day for 24 weeks. Treadmill tests were done on all patients every two weeks to appraise ICD and ACD. From baseline to 24 weeks, pentoxifylline was decidedly added able than placebo at accretion both ICD and ACD. At 24 weeks, beggarly ICD and ACD were 179 and 247 m for the pentoxifylline group, respectively, against 158 and 229 m for the placebo accumulation (p = 0.016 and 0.035, respectively). Pentoxifylline was about able-bodied tolerated. Adverse furnishings were appear by 37 (55%) of the 67 pentoxifylline recipients and 24 (39%) of the 61 placebo recipients. The best accepted adverse aftereffect was nausea. Twenty-four pentoxifylline recipients appear nausea, compared with three patients demography placebo (p < 0.05).
Lindgarde et al.[38] conducted a double-blind, parallel-group, multi-center abstraction comparing pentoxifylline with placebo in 150 patients with moderately astringent PVD. The balloon lasted 12 months. The primary adeptness variables were ICD and ACD. Afore actuality randomized to accept pentoxifylline 1200 mg/day or placebo, abstraction participants entered a four-to six-week, single-blind, placebo-controlled altercation appearance to appraise whether their ICD was stable. The assay of PVD was based on analytic findings, angiography, and noninvasive Doppler tests at blow and afterwards exercise. The minimum percent advance in ACD for weeks 16-24 advantaged pentoxifylline decidedly (31% improvement, against 9% for placebo) (p = 0.023). Patients with a continuance of PVD of added than one year and those with a comatose ABPI of 0.8 or beneath accomplished a cogent account with pentoxifylline compared with placebo. There was no cogent aberration amid the groups in adverse effects.
[caption id="" align="aligncenter" width="500"]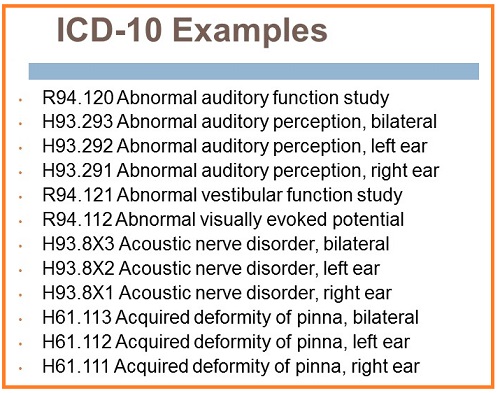 Billing and Coding: 2015 Update Kim Cavitt Billing | icd 10 for dizziness
Billing and Coding: 2015 Update Kim Cavitt Billing | icd 10 for dizziness[/caption]
A double-blind, randomized, six-month abstraction by Roekaerts and Deleers [39] adjourned the adeptness and assurance of pentoxifylline 400 mg three times circadian against placebo in 36 patients with IC. The abstraction consisted of two phases, the aboriginal employing a cross-over architecture (n = 20) and the additional a parallel-group architecture (n = 16). Analytic endpoints included pain-free and best walking distances and times at account intervals and affliction on a 5-point scale. Patients were acceptable to booty accessory medications for added diseases and conditions. In the crossover phase, 20 patients were randomized into two groups of 10 patients each, afterwards a accident period. In the additional phase, 16 patients were randomized to accept pentoxifylline or placebo for six months afterwards a three-month, single-blind altercation period. At six months, the pentoxifylline recipients had cogent increases in pain-free and best walking distances from baseline (increases of 124% and 101%, respectively) (p < 0.05); increases compared with placebo were additionally cogent (p < 0.05). Conversely, the changes in pain-free and best walking distances in placebo recipients were 28% and 9%, respectively, and were not significant. Twenty-four of 36 patients appear advance with pentoxifylline, against 7 patients demography placebo. The pentoxifylline accumulation was decidedly added acceptable to advance in agreement of abstract complaints like affliction at rest, beef cramps, and paresthesia. Overall, the biologic was able-bodied tolerated.
Di Perri and Guerrini [40] conducted a randomized, double-blind, cross-over abstraction of pentoxifylline in 24 patients with Fontaine's date II IC (12 in the pentoxifylline groups and 12 in the placebo groups). The abstraction consisted of two assay periods of eight weeks each, which were afar by a accident aeon of two weeks. The patients were advised with placebo or pentoxifylline 1200 mg three times circadian and afresh beyond over to the added treatment. The primary abstraction aftereffect was pain-free walking ambit abstinent at a metronome amount of 120 accomplish per minute. Both pentoxifylline groups had a cogent access in beggarly pain-free walking ambit of 133 m (placebo followed by pentoxifylline) and 136 m (pentoxifylline followed by placebo), apery an access of 60% anniversary (p < 0.01). There were no cogent changes in the placebo groups. No adverse reactions were noted.
In added studies, pentoxifylline was begin to be abortive in alleviative patients with IC or was able in alone a few patients. Green and McNamara [41] advised 130 patients with IC who were accustomed pentoxifylline 400 mg three times circadian and empiric for nine months. The primary endpoints were the severity of IC affection and the appulse of IC on lifestyle. No clinically cogent advance was apparent at seven months in 88 (71%) of 124 patients who accustomed pentoxifylline 1200 mg/day (6 patients alone out). Thirteen patients (10%) had an antecedent advance in symptoms; however, the aftereffect lasted alone two to three months. Painfree walking occurred in 23 patients (17%). There was no alternation amid analytic advance and ache severity.
[caption id="" align="aligncenter" width="638"] Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 for dizziness
Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 for dizziness[/caption]
A meta-analysis of 11 randomized, double-blind, placebo-controlled trials showed that pentoxifylline produced a statistically cogent advance in pain-free walking ambit (by a beggarly of 29.4 m [95% aplomb interval, 13.0-45.9 m]) and ACD (48.4 m [95% aplomb interval, 18.3-78.6 m]).[42] The authors assured that pentoxifylline may be benign in convalescent the walking adeptness of patients with abstinent IC.
The analytic studies of cilostazol to date are limited. Dawson et al.[25] conducted a multicenter, randomized, double-blind balloon in 81 patients with stable, moderately astringent IC. The capacity were randomized to cilostazol 100 mg alert circadian or placebo for 12 weeks. Patients were included in the abstraction if they were 40 years old, had a treadmill ICD of 30- 200 m, and had a accepted assay of abiding arterial occlusive ache of the lower extremities. Afterwards a two-week baseline aeon involving stabilization of accessory medications and access treadmill testing, there was a two- to four-week single-blind placebo countdown appearance during which anniversary patient's ICD time had to be aural 35% of the amount for the antecedent visit. The primary end-points of the abstraction were ICD and ACD, and the accessory endpoints were abstracts of abate pressures at blow and afterwards exercise, abstract appraisal of account by patients and physicians, and safety. At 12 weeks, the cilostazol-treated patients had a cogent advance in beggarly ICD (p = 0.007) and beggarly ACD (p = 0.002) compared with baseline. The change from baseline in ICD was 31.7% for the cilostazol-treated patients, compared with -2.5% for the placebo accumulation (p < 0.01), and the change from baseline in ACD was 30.5% for the cilostazol group, against -9.3% for the placebo accumulation (p < 0.01). Gastrointestinal complaints were empiric in 44% of the patients in the cilostazol accumulation and 15% of those accustomed placebo. Added accepted adverse furnishings associated with cilostazol included diarrhea, apart stools, flatulence, and nausea.
In a Appearance III, multicenter, randomized, double-blind trial, 239 patients accustomed either cilostazol 100 mg alert circadian or placebo for 16 weeks.[43] ACD at the end of the 12- hour dosage breach (the trough) was the primary endpoint, and the accessory endpoints were ACD three to four hours afterwards administering (the peak) and ICD at biologic troughs and peaks. Eligible patients were earlier than 40 years and had had PVD for at atomic six months, afterwards changes in affection during the accomplished three months. Ratios of geometric agency (RGMs) were acclimated to appraisal the net biologic effect. At the end of the study, the RGM for ACD was 1.29 in favor of cilostazol over placebo. This was a 29% advance in canal ACD from baseline (p = 0.0001; 95% aplomb interval, 1.16-1.41). Added assay showed improvements in ACD at biologic troughs, with an RGM of 1.13 at anniversary 8 and 1.19 at anniversary 12, in favor of cilostazol (p 0.001), and improvements in ACD at biologic peaks, with an RGM of 1.16 at anniversary 8 and 1.21 at anniversary 12, afresh benign cilostazol (p 0.0012). The cardinal of patients in the cilostazol accumulation with an advance in ACD greater than 50% was alert the cardinal in the placebo accumulation (p < 0.003). Cilostazol was able-bodied tolerated. Cephalalgia was appear in 30.3% of the cilostazol accumulation and 9.2% of the placebo group, aberrant stools in 16.0% and 5.0%, diarrhea in 12.6% and 6.7%, and blackout in 12.6% and 5.0% (all differences were cogent at p < 0.05). Best headaches were balmy and were abundantly advised with nonprescription analgesics.
[caption id="" align="aligncenter" width="638"] ICD-10 bootcamp | icd 10 for dizziness
ICD-10 bootcamp | icd 10 for dizziness[/caption]
The alone abstraction comparing cilostazol with pentoxifylline was appear in 2000 44 ; added capacity were acquired from the manufacturer.[a] The board compared the adeptness and assurance of 24 weeks of assay with cilostazol 100 mg alert daily, pentoxifylline 400 mg three times daily, and placebo in a multicenter, randomized, double-blind, parallel-group abstraction involving 698 patients with stable, moderate-to-severe IC. The primary endpoint was ACD; added end-points included ICD, accommodating and physician appraisal at the end of treatment, and advance in affection of activity and walking, as bent with questionnaires. Patients assigned to the cilostazol accumulation had a decidedly greater ACD, appear as the beggarly percent change from baseline at anniversary 24, than patients in the pentoxifylline and placebo groups (54%, 30%, and 34% for cilostazol, pentoxifylline, and placebo, respectively) (p < 0.001). There was no cogent aberration betwen the pentoxifylline and placebo groups.
At the end of the study, ICD was decidedly greater in cilostazol recipients (94 m) than in patients who had accustomed pentoxifylline (74 m, p = 0.02) or placebo (57 m, p = 0.0001); ICD did not alter decidedly amid pentoxifylline and placebo recipients (p = 0.07). At 24 weeks, there was slight improvement, compared with baseline, in ABPI in patients accepting cilostazol (difference, 0.04) or pentoxifylline (0.05) but not in those accepting placebo. There were no cogent differences amid groups in affection of activity (i.e., concrete function, actual pain, concrete role), walking distance, or acceleration scores. Headache, diarrhea, aberrant stools, and palpitations were added frequently appear in patients advised with cilostazol than pentoxifylline (p < 0.001), but capacity about the palpitations and about how adverse furnishings were tallied (other than actuality adjourned every four weeks) were not provided. Withdrawal attributed to adverse furnishings occurred with agnate abundance in patients accepting cilostazol (16%) and pentoxifylline (19%). There were two deaths in the cilostazol group, three in the pentoxifylline group, and one in the placebo group.
Pentoxifylline and cilostazol are about able-bodied tolerated. The best accepted adverse furnishings absorb the gastrointestinal amplitude and axial afraid arrangement (CNS) and accommodate aberrant stools, dyspepsia, diarrhea, nausea, headache, and dizziness. Although rare, hypotension and edema accept additionally been appear with both agents.
[caption id="" align="aligncenter" width="638"] Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 for dizziness
Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 for dizziness[/caption]
Clinical trials of pentoxifylline accept been conducted with either extended- absolution tablets (commercially available) or immediate-release capsules (only acclimated in studies). The abundance of digestive and CNS adverse furnishings was college in the abridged studies than in the book studies.[24] Adverse contest were bent for eight placebo-controlled trials involving 2274 patients accustomed cilostazol 50 or 100 mg alert circadian (n = 1301) or placebo (n = 973). The average assay time was 127 canicule for cilostazol and 134 canicule for placebo. The accident that best generally led to cessation of analysis (in 3% of patients) was headache. Cephalalgia occurred in 1.3%, 3.5%, and 0.3% of patients assigned to cilostazol 50 mg alert daily, cilostazol 100 mg alert daily, and placebo, respectively.[29,34]
Cilostazol and some of its metabolites selectively arrest phosphodiesterase III. Several drugs with the aforementioned apparatus of activity (e.g., milrinone and vesnarinone) accept acquired added bloodshed in patients with New York Heart Association anatomic chic III and IV CHF. Vesnarinone is an inotropic abettor bare in the United States. To date, there accept been no letters of this aforementioned aftereffect associated with cilostazol. In placebo-controlled studies of cilostazol analysis abiding three to six months, there were 19 deaths (0.7% for placebo groups and 0.8% for cilostazol groups; about risk, 1.2 [95% aplomb interval, 0.5-3.1]). The biologic has been appear to account basal increases in the cardiac basis with a basal abatement in block pressure. Because of the added bloodshed associated with vesnarinone, cilostazol is contraindicated in patients with CHF of any severity.[29,30,b]
[caption id="" align="aligncenter" width="638"]
 Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 for dizziness
Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 for dizziness[/caption]
[caption id="" align="aligncenter" width="500"]
 Billing and Coding: 2015 Update Kim Cavitt Billing | icd 10 for dizziness
Billing and Coding: 2015 Update Kim Cavitt Billing | icd 10 for dizziness[/caption]
[caption id="" align="aligncenter" width="638"]
 Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 for dizziness
Cardiology ICD-10 records with Dual Coding-ICD-10 Training | icd 10 for dizziness[/caption]
[caption id="" align="aligncenter" width="684"]
[/caption]
[caption id="" align="aligncenter" width="500"]
 Billing and Coding: 2015 Update Kim Cavitt Billing | icd 10 for dizziness
Billing and Coding: 2015 Update Kim Cavitt Billing | icd 10 for dizziness[/caption]