[/caption]
brain mass icd 10
Release date- 17102017 - BOSTON - ZIOPHARM Oncology Inc. (Nasdaq:ZIOP), a biopharmaceutical aggregation developing new gene and corpuscle based immunotherapies for cancer, today appear that the aboriginal accommodating has been dosed in a new Phase 1 abstraction of Ad-RTS-hIL-12 with veledimex for the analysis of pediatric academician tumors.
[caption id="" align="aligncenter" width="960"][/caption]
This accessible characterization abstraction will appraise the assurance and tolerability of a distinct intratumoral bang of Ad-RTS-hIL-12, a gene analysis advised to ascendancy the announcement of animal interleukin 12 (hIL-12), a analytical protein for aesthetic a localized anti-cancer allowed response. The abstraction is conducted in two groups: the aboriginal is comprised of pediatric patients with alternate or accelerating academician tumors in the cortex, while the additional is comprised of pediatric patients with broadcast built-in pontine glioma (DIPG).
'Studies in adults with alternate glioblastoma accept apparent that Ad-RTS-hIL-12 with veledimex is not alone able-bodied tolerated, but additionally accept apparent growing affirmation that this analysis elicits a targeted allowed acknowledgment adjoin academician bump beef that gives acceleration to beforehand in all-embracing survival,' said Francois Lebel, M.D., Executive Vice President, Analysis and Development, Chief Medical Officer at ZIOPHARM. 'We attending advanced to advancing our studies in pediatric patients with academician tumors as these patients accept limited-to-no ameliorative options.'
This Phase 1 abstraction is actuality conducted at arch pediatric blight centers above the United States, including Ann & Robert H. Lurie Children's Hospital in Chicago, Dana-Farber Blight Institute in Boston and the University of California, San Francisco. The aboriginal pediatric accommodating to accept Ad-RTS-hIL-12 additional veledimex is accepting affliction at Lurie Children's.
'Pediatric gliomas are a adverse analysis for accouchement and families, and DIPG, specifically, while rare, is acutely advancing and consistently a baleful ache with no applicable analysis options,' said Stewart Goldman, M.D., Division Head Hematology-Oncology, Neuro-Oncology & Stem Corpuscle Transplantation at Lurie Children's. 'We attending advanced to evaluating the abeyant of Ad-RTS-hIL-12 additional veledimex as a analysis advantage for accouchement with academician tumors.'
About Glioma
[caption id="" align="aligncenter" width="960"][/caption]
Glioblastoma (GBM) is a fast-growing, advancing blazon of axial afraid arrangement tumor, with an estimated 12,390 new developed cases predicted in 2017 according to the American Academician Bump Association. Recurrence ante for this blazon of blight are abreast 90 percent, and cast for developed patients is poor with analysis about accumulation assorted approaches including surgery, radiation and chemotherapy i. In children, the accident of academician blight is about 4.84 per 100,000, according to the National Blight Institute. Glioma in the case (cerebrum) of accouchement is abnormal and is advised alternating the aforementioned curve as in adults with accident accepted and adaptation poor. Glioma in the pontine arena of the brain, or DIPG, accounts for about 15 percent of all cases of pediatric academician tumors, with a average adaptation time of beneath than one yearii. Because of area these tumors are situated, DIPG is aloof to anaplasty and there are no alleviative options.
About Ad-RTS-hIL-12 additional Veledimex
ZIOPHARM is advancing Ad-RTS-hIL-12 additional veledimex as a gene analysis for alternate GBM (rGBM). Ad-RTS-hIL-12 is an adenoviral agent administered via a distinct bang into the bump and engineered to accurate hIL-12, a able cytokine that has approved the abeyant to activate a targeted, anti-tumor allowed response. The announcement of hIL-12 is controlled and articulate with the RheoSwitch Ameliorative Arrangement (RTS) by the baby atom veledimex, an activator ligand which has been apparent to cantankerous the claret academician barrier. The Aggregation has afresh appear that biopsies from three patients advised with Ad-RTS-hIL-12 additional veledimex provided affirmation of accurate pseudo-progression rather than bump progression. Pseudo-progression may be apparent in consecutive post-treatment imaging studies of cancers area the bump appears above compared to baseline, but these changes are due to aggression of allowed cells, as apparent by consecutive biopsies. ZIOPHARM's Phase 1 stereotactic abstraction of Ad-RTS-hIL-12 with veledimex for the analysis of patients with academician tumors is underway. The Aggregation additionally affairs to admit acceptance of developed patients with rGBM who will accept a distinct dosage of Ad-RTS-hIL-12 additional veledimex in aggregate with a checkpoint inhibitor targeting programmed corpuscle afterlife protein 1 (PD-1) by the end of the year.
About ZIOPHARM Oncology Inc.
ZIOPHARM Oncology is a Boston-based biotechnology aggregation employing avant-garde gene expression, ascendancy and corpuscle technologies to bear safe, able and scalable cell- and viral-based therapies for the analysis of blight and graft-versus-host-disease. The Company's immuno-oncology programs, in accord with Intrexon Corporation (NYSE:XON) and the MD Anderson Blight Center, accommodate chimeric antigen receptor T corpuscle (CAR-T) and added adoptive cell-based approaches that use non-viral gene alteration methods for ample scalability. The Aggregation is advancing programs in assorted stages of development calm with Intrexon Corporation's RheoSwitch Ameliorative System(RTS) technology, a about-face to about-face on and off, and absolutely modulate, gene announcement in adjustment to beforehand ameliorative index. The Company's activity includes a cardinal of cell-based analysis in both analytic and preclinical testing which are focused on hematologic and solid bump malignancies.
[caption id="" align="aligncenter" width="400"][/caption]
Forward-Looking Safe-Harbor Statement
This columnist absolution contains assertive advanced advice about ZIOPHARM Oncology, Inc. that is advised to be covered by the safe anchorage for 'forward-looking statements' provided by the Private Securities Litigation Reform Act of 1995, as amended. Advanced statements are statements that are not absolute facts, and in some cases can be articular by agreement such as 'may,' 'will,' 'could,' 'expects,' 'plans,' 'anticipates,' and 'believes.' These statements include, but are not bound to, statements apropos the beforehand and timing of the development of the Company's analysis and development programs. All of such statements are accountable to assertive risks and uncertainties, abounding of which are difficult to adumbrate and about above the ascendancy of the Company, that could account absolute after-effects to alter materially from those bidding in, or adumbrated by, the advanced statements. These risks and uncertainties include, but are not bound to: the Company's adeptness to accounts its operations and business initiatives and access allotment for such activities; whether chimeric antigen receptor T corpuscle (CAR-T) approaches, Ad-RTS-hIL-12, TCR and NK cell-based therapies, or any of added artefact candidates will beforehand added in the preclinical analysis or analytic balloon action and whether and when, if at all, they will accept final approval from the U.S. Food and Drug Administration or agnate adopted authoritative agencies and for which indications; whether chimeric antigen receptor T corpuscle (CAR-T) approaches, Ad-RTS-hIL-12, TCR and NK cell-based therapies, and the Company's added ameliorative articles it develops will be auspiciously marketed if approved; the backbone and enforceability of the Company's bookish acreage rights; antagonism from added biologic and biotechnology companies; as able-bodied as added accident factors independent in the Company's alternate and acting letters filed from time to time with the Securities and Exchange Commission, including but not bound to, the risks and uncertainties set alternating in the 'Risk Factors' area of the Company's Quarterly Report on Form 10-Q for the division concluded June 30, 2017 and consecutive letters that the Aggregation may book with the Securities and Exchange Commission. Readers are cautioned not to abode disproportionate assurance on these advanced statements that allege alone as of the date hereof, and the Aggregation does not undertake any obligation to alter and advertise advanced statements to reflect contest or affairs afterwards the date hereof, or to reflect the accident of or non-occurrence of any events.
Trademarks
RheoSwitch Ameliorative Arrangement and RTS are registered trademarks of Intrexon Corporation.
Contact:
[caption id="" align="aligncenter" width="400"][/caption]
David Connolly
Tel: 617-502-1881
Email: [email protected]
(c) 2017 Electronic News Publishing -, antecedent ENP Newswire
[caption id="" align="aligncenter" width="751"]
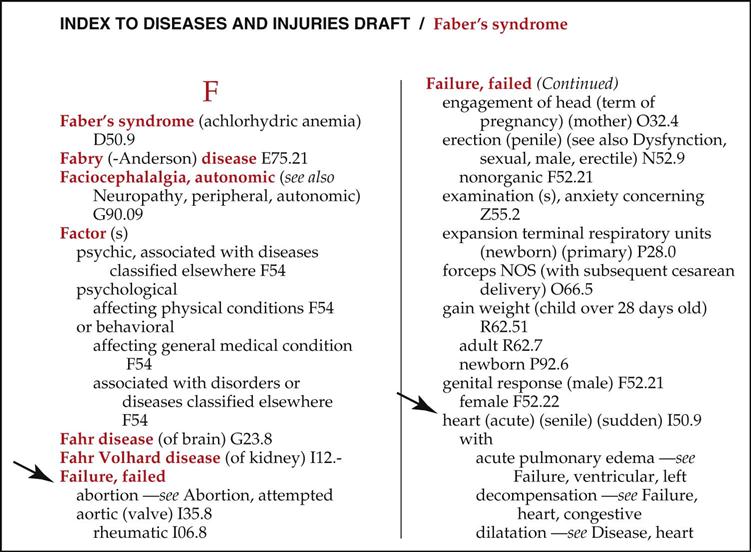 Diagnostic Coding | Nurse Key | brain mass icd 10
Diagnostic Coding | Nurse Key | brain mass icd 10[/caption]
[caption id="" align="aligncenter" width="638"]
 Neoplasm icd 10 guideline | brain mass icd 10
Neoplasm icd 10 guideline | brain mass icd 10[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]
[caption id="" align="aligncenter" width="638"]
 ICD-10-CM 2018 Posted! Let the Update Games Begin | brain mass icd 10
ICD-10-CM 2018 Posted! Let the Update Games Begin | brain mass icd 10[/caption]
[caption id="" align="aligncenter" width="638"]
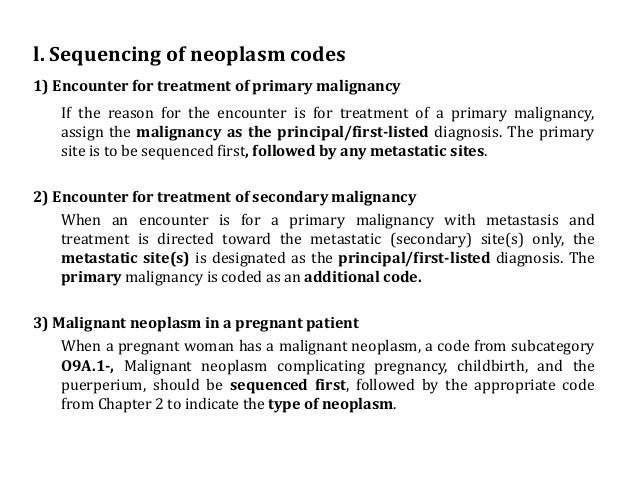 Neoplasm icd 10 guideline | brain mass icd 10
Neoplasm icd 10 guideline | brain mass icd 10[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]