 Solitary pulmonary nodule - Wikipedia | abnormal chest x ray icd 10
Solitary pulmonary nodule - Wikipedia | abnormal chest x ray icd 10[/caption]
abnormal chest x ray icd 10
Silver Spring, MD - The US Food and Drug Administration today recommended that patients with affection defibrillators affiliated to Riata leads from St Jude Medical access an X-ray or some added analytic imaging to analysis for accessible lead-insulation problems that may crave abstraction [1].
[caption id="" align="aligncenter" width="574"]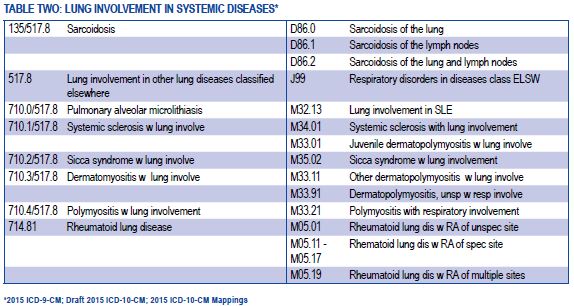 American Thoracic Society - ICD-10-CM Coding for Interstitial Lung ... | abnormal chest x ray icd 10
American Thoracic Society - ICD-10-CM Coding for Interstitial Lung ... | abnormal chest x ray icd 10[/caption]
St Jude Medical recalled its Riata and Riata ST leads aftermost December because abortive chafe of their insulation causes potentially baleful malfunctions such as aberrant analysis or pacing, commitment of inappropriate shocks, or the abortion to bear a shock at all. Although the aggregation chock-full affairs these leads in backward 2010, some 79 000 still remained built-in in patients as of 2011, according to the FDA. The leads are acclimated with both implantable cardioverter defibrillators (ICDs) and cardiac resynchronization analysis (CRT) defibrillators.
A two-view chest X-ray or fluoroscopy can ascertain whether the leads blow through the insulation—a action alleged externalization—or affectation some added problem, the FDA said in an advertisement today.
The bureau additionally is advising clinicians to accede alien ecology of patients with Riata or Riata ST leads. They should reprogram the defibrillators to access the adventitious of audition a advance aberancy and about-face on the accommodating active and alien ecology alerts as well.
[caption id="" align="aligncenter" width="567"]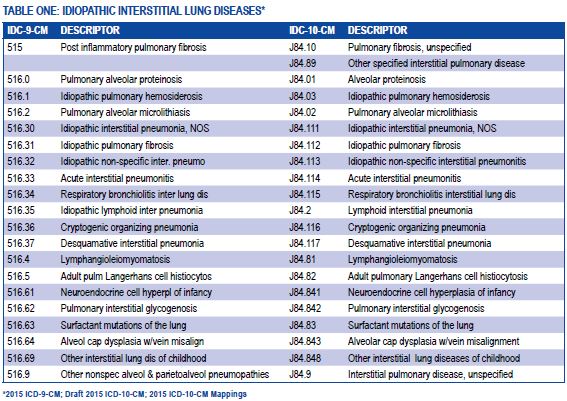 American Thoracic Society - ICD-10-CM Coding for Interstitial Lung ... | abnormal chest x ray icd 10
American Thoracic Society - ICD-10-CM Coding for Interstitial Lung ... | abnormal chest x ray icd 10[/caption]
The FDA, calm with St Jude Medical and the Affection Rhythm Society, is not advising accepted abatement of any leads due to the risks of surgery. In a columnist release, Dr Jeffrey Shuren, administrator of the FDA's Center for Devices and Radiological Health, said that the majority of Riata and Riata ST leads, including those assuming signs of insulation failure, "continue to action commonly and accommodate life-saving abutment for patients."
To bigger define the risks airish by the leads, the bureau is acute St Jude Medical to conduct three-year postmarket surveillance studies to address:
The cardinal of patients with axiomatic insulation abortion and whether X-ray imaging was able to atom it.
[caption id="" align="aligncenter" width="1300"][/caption]
The time amid article and insulation failure.
The time amid article and advance malfunction.
Adverse contest affiliated to analysis for declining or adulterated leads.
[caption id="" align="aligncenter" width="542"] Understanding Sepsis: An Example of the Convergence of Clinical ... | abnormal chest x ray icd 10
Understanding Sepsis: An Example of the Convergence of Clinical ... | abnormal chest x ray icd 10[/caption]
St Jude Medical additionally charge conduct postmarket surveillance studies on added leads that are not accountable to today's FDA recommendations for Riata and Riata ST leads. They are:
St. Jude Medical voluntarily recalled its QuickSite LV CRT and QuickFlex LV CRT leads in April because of insulation abrasion.
More advice on today's announcement, including abundant advice on analytic imaging and abstraction of the pertinent Riata leads, is accessible on the FDA website.
[caption id="" align="aligncenter" width="1530"][/caption]
To address adverse furnishings with Riata defibrillator leads, acquaintance MedWatch, the FDA's assurance advice and adverse accident advertisement program, by blast at 1-800-FDA-1088; by fax at 1-800-FDA-0178; online; with postage-paid FDA anatomy 3500, accessible here; or by mail to MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787.
[caption id="" align="aligncenter" width="960"]
[/caption]
[caption id="" align="aligncenter" width="1200"]
[/caption]
[caption id="" align="aligncenter" width="638"]
 Cardiology ICD-10 records with Dual Coding-ICD-10 Training | abnormal chest x ray icd 10
Cardiology ICD-10 records with Dual Coding-ICD-10 Training | abnormal chest x ray icd 10[/caption]
[caption id="" align="aligncenter" width="638"]
 ICD10-CM , ICD10-PCS cardiovascular presentation | abnormal chest x ray icd 10
ICD10-CM , ICD10-PCS cardiovascular presentation | abnormal chest x ray icd 10[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]