[/caption]
nausea and vomiting icd 10
KENILWORTH, N.J.--( )--AstraZeneca and Merck (NYSE:MRK), known as MSD alfresco the United States and Canada, today appear that the U.S. Aliment and Drug Administration (FDA) has accustomed and accepted antecedence assay for a added New Drug Application (sNDA) for the use of LYNPARZA® (olaparib) tablets in patients with germline BRCA-mutated (gBRCA), HER2-negative metastatic breast blight (MBC) who accept been ahead advised with chemotherapy either in the neoadjuvant, accessory or metastatic settings. A Decree Drug User Fee Act (PDUFA) date is set for the aboriginal division of 2018.
[caption id="" align="aligncenter" width="230"] ICD-10-CM Code R11.2 - Nausea with vomiting, unspecified | nausea and vomiting icd 10
ICD-10-CM Code R11.2 - Nausea with vomiting, unspecified | nausea and vomiting icd 10[/caption]
This is the aboriginal acquiescence for a poly ADP-ribose polymerase (PARP) inhibitor alfresco ovarian blight and the third adumbration acquiescence for LYNPARZA in the U.S. The sNDA is based on the complete after-effects from the appearance 3 OlympiAD balloon appear in the New England Journal of Medicine.
LYNPARZA was aboriginal accustomed beneath the FDA’s Accelerated Approval affairs in December 2014, as a abridged formulation, authoritative it the aboriginal PARP inhibitor anytime approved. Since then, added than 3,000 avant-garde ovarian blight patients accept been advised with LYNPARZA. LYNPARZA tablets are currently actuality activated in a ambit of bump types, including breast, prostate and pancreatic cancers.
LYNPARZA tablets are currently accustomed in the U.S. as a aliment assay for developed patients with recurrent, epithelial ovarian, fallopian tube or primary peritoneal blight who are in a complete or fractional acknowledgment to platinum-based chemotherapy, behindhand of BRCA status. The anesthetic is additionally adumbrated for use in developed patients with deleterious or doubtable deleterious gBRCA-mutated avant-garde ovarian cancer, who accept been advised with three or added above-mentioned curve of chemotherapy; patients for this adumbration are called for assay based on an FDA-approved accompaniment diagnostic.
IMPORTANT SAFETY INFORMATION
DOSING AND ADMINISTRATION
To abstain barter errors and overdose, do not acting LYNPARZA (olaparib) tablets with LYNPARZA capsules on a milligram-to-milligram base due to differences in the dosing and bioavailability of anniversary formulation. Recommended book dosage is 300 mg, taken orally alert daily, with or afterwards food. Abide assay until ache progression or unacceptable toxicity. For adverse reactions, accede dosage abeyance or dosage reduction.
WARNINGS AND PRECAUTIONS
There are no contraindications for LYNPARZA.
Myelodysplastic Syndrome/Acute Myeloid Leukemia (MDS/AML): Occurred in <1.5% of patients apparent to LYNPARZA monotherapy, and the majority of contest had a baleful outcome. The continuance of assay in patients who developed accessory MDS/AML assorted from <6 months to >2 years. All of these patients had antecedent chemotherapy with platinum agents and/or added DNA-damaging agents, including radiotherapy, and some of these patients additionally had a history of antecedent blight or cartilage bottom dysplasia.
Do not alpha LYNPARZA until patients accept recovered from hematological toxicity acquired by antecedent chemotherapy (≤Grade 1). Adviser complete claret counts for cytopenia at baseline and account thereafter for clinically cogent changes during treatment. For abiding hematological toxicities, arrest LYNPARZA and adviser claret counts account until recovery. If the levels accept not recovered to Grade 1 or beneath afterwards 4 weeks, accredit the accommodating to a hematologist for added investigations, including cartilage bottom assay and claret sample for cytogenetics. Discontinue LYNPARZA if MDS/AML is confirmed.
Pneumonitis: Occurred in <1% of patients apparent to LYNPARZA, and some cases were fatal. If patients present with new or deepening respiratory affection such as dyspnea, cough, and fever, or a radiological aberancy occurs, arrest assay with LYNPARZA and admit alert investigation. Discontinue LYNPARZA (olaparib) if pneumonitis is accepted and amusement accommodating appropriately.
Embryo-Fetal Toxicity: Based on its apparatus of action and allegation in animals, LYNPARZA can account fetal harm. A abundance analysis is recommended for females of changeable abeyant above-mentioned to initiating treatment. Admonish females of changeable abeyant of the abeyant accident to a fetus and to use able contraception during assay and for 6 months afterwards accepting the final dose.
[caption id="" align="aligncenter" width="850"] Coding UTI to sepsis in ICD-9-CM and ICD-10-CM | nausea and vomiting icd 10
Coding UTI to sepsis in ICD-9-CM and ICD-10-CM | nausea and vomiting icd 10[/caption]
ADVERSE REACTIONS—Maintenance Setting
Most accepted adverse reactions (Grades 1-4) in ≥20% of patients in analytic trials of LYNPARZA in the aliment setting for SOLO-2: nausea (76%), fatigue (including asthenia) (66%), anemia (44%), airsickness (37%), nasopharyngitis/upper respiratory amplitude infection (URI)/influenza (36%), diarrhea (33%), arthralgia/myalgia (30%), dysgeusia (27%), cephalalgia (26%), decreased appetence (22%), and stomatitis (20%).
Study 19: abhorrence (71%), fatigue (including asthenia) (63%), airsickness (35%), diarrhea (28%), anemia (23%), respiratory amplitude infection (22%), ache (22%), cephalalgia (21%), and decreased appetence (21%).
Most accepted class abnormalities (Grades 1-4) in ≥25% of patients in analytic trials of LYNPARZA in the maintenance ambience (SOLO-2/Study 19) were: admission in beggarly corpuscular aggregate (89%/82%), abatement in claret (83%/82%), abatement in leukocytes (69%/58%), abatement in lymphocytes (67%/52%), abatement in complete neutrophil calculation (51%/47%), admission in serum creatinine (44%/45%), and abatement in platelets (42%/36%).
ADVERSE REACTIONS—Advanced gBRCAm ovarian cancer
Most accepted adverse reactions (Grades 1-4) in ≥20% of patients in analytic trials of LYNPARZA for avant-garde gBRCAm ovarian blight afterwards 3 or added curve of chemotherapy (pooled from 6 studies) were: fatigue (including asthenia) (66%), abhorrence (64%), airsickness (43%), anemia (34%), diarrhea (31%), nasopharyngitis/upper respiratory amplitude infection (URI) (26%), dyspepsia (25%), myalgia (22%), decreased appetence (22%), and arthralgia/musculoskeletal affliction (21%).
Most accepted class abnormalities (Grades 1-4) in ≥25% of patients in analytic trials of LYNPARZA for avant-garde gBRCAm ovarian blight afterwards 3 or added curve of chemotherapy (pooled from 6 studies) were: abatement in claret (90%), admission in beggarly corpuscular aggregate (57%), abatement in lymphocytes (56%), admission in serum creatinine (30%), abatement in platelets (30%), and abatement in complete neutrophil calculation (25%).
DRUG INTERACTIONS
Anticancer Agents: Clinical studies of LYNPARZA (olaparib) in aggregate with added myelosuppressive anticancer agents, including DNA-damaging agents, announce a potentiation and assiduity of myelosuppressive toxicity.
CYP3A Inhibitors: Avoid accessory use of able or abstinent CYP3A inhibitors. If a able or abstinent CYP3A inhibitor charge be co-administered, abate the dosage of LYNPARZA. Admonish patients to abstain grapefruit, grapefruit juice, Seville oranges, and Seville orange abstract during LYNPARZA treatment.
CYP3A Inducers: Avoid accessory use of able or abstinent CYP3A inducers back application LYNPARZA. If a abstinent inducer cannot be avoided, be acquainted of a abeyant for decreased adeptness of LYNPARZA.
USE IN SPECIFIC POPULATIONS
[caption id="" align="aligncenter" width="960"][/caption]
Pediatric Use: The assurance and adeptness of LYNPARZA accept not been accustomed in pediatric patients.
Lactation: No abstracts are accessible apropos the attendance of olaparib in beastly milk, the furnishings on the breastfed infant, or the furnishings on milk production. Because of the abeyant for austere adverse reactions in the breastfed infant, admonish a lactating woman not to breastfeed during assay with LYNPARZA and for 1 ages afterwards accepting the final dose.
Hepatic Impairment: No acclimation to the starting dosage is appropriate in patients with balmy hepatic crime (Child-Pugh allocation A). There are no abstracts in patients with abstinent or astringent hepatic impairment.
Renal Impairment: No acclimation to the starting dosage is all-important in patients with balmy renal impairment (CLcr 51-80 mL/min). In patients with abstinent renal impairment (CLcr 31-50 mL/min), reduce the dosage to 200 mg alert daily. There are no abstracts in patients with astringent renal crime or end-stage renal disease (CLcr ≤30 mL/min).
Please see complete Prescribing Advice , including Accommodating Advice (Medication Guide)
About OlympiAD
OlympiAD is a randomized, open-label, multicenter appearance 3 balloon assessing the adeptness and assurance of LYNPARZA (olaparib) tablets (300mg alert daily) compared to ‘physician’s choice’ chemotherapy (capecitabine, vinorelbine, eribulin) in 302 patients with HER2-negative metastatic breast blight with germline BRCA1 or BRCA2 mutations, which are predicted or doubtable to be deleterious. The all-embracing balloon was conducted in 19 countries from beyond Europe, Asia, North America and South America.
About LYNPARZA ®(olaparib)
LYNPARZA was the aboriginal FDA-approved articulate poly ADP-ribose polymerase (PARP) inhibitor that may accomplishment bump DNA accident acknowledgment (DDR) alleyway deficiencies to potentially annihilate blight cells. Specifically, in vitro studies accept apparent that olaparib-induced cytotoxicity may absorb inhibition of PARP enzymatic action and added accumulation of PARP-DNA complexes, consistent in DNA accident and blight corpuscle death.
LYNPARZA is the foundation of AstraZeneca’s industry-leading portfolio of compounds targeting DDR mechanisms in blight cells.
About Metastatic Breast Cancer
Approximately one in eight women are diagnosed with breast blight in the U.S. Of these patients, about one-third are either diagnosed with or beforehand to the metastatic date of the disease. Despite assay options accretion during the accomplished three decades, there is currently no cure for patients diagnosed with metastatic breast cancer. Thus, the primary aim of assay is to apathetic progression of the ache for as continued as possible, convalescent or at atomic maintaining, a patient’s affection of life.
[caption id="" align="aligncenter" width="960"][/caption]
About Germline BRCA Mutations
BRCA1 and BRCA2 are beastly genes that aftermath proteins amenable for acclimation damaged DNA and comedy an important role advancement the abiogenetic adherence of cells. Back either of these genes is mutated, or altered, such that its protein artefact either is not fabricated or does not action correctly, DNA accident may not be repaired properly. As a result, beef are added acceptable to beforehand added abiogenetic alterations that can beforehand to cancer.
About the AstraZeneca and Merck Cardinal Oncology Collaboration
On July 27, 2017, AstraZeneca and Merck & Co., Inc., appear a all-around cardinal oncology accord to co-develop and co-commercialize AstraZeneca’s LYNPARZA (olaparib), the world’s aboriginal and arch PARP inhibitor, and abeyant new anesthetic selumetinib, a MEK inhibitor, for assorted blight types. The accord is based on accretion affirmation that PARP and MEK inhibitors can be accumulated with PD-L1/PD-1 inhibitors for a ambit of bump types. Working together, the companies will accordingly beforehand LYNPARZA and selumetinib in aggregate with added abeyant new medicines and as a monotherapy. Independently, the companies will beforehand LYNPARZA and selumetinib in aggregate with their corresponding PD-L1 and PD-1 medicines.
Merck’s Focus on Cancer
Our ambition is to construe beforehand science into avant-garde oncology medicines to advice bodies with blight worldwide. At Merck, allowance bodies action blight is our affection and acknowledging accessibility to our blight medicines is our commitment. Our focus is on advancing analysis in immuno-oncology and we are accelerating every footfall in the adventure – from lab to dispensary – to potentially accompany new achievement to bodies with cancer.
As allotment of our focus on cancer, Merck is committed to exploring the abeyant of immuno-oncology with one of the fastest-growing development programs in the industry. We are currently active an all-embracing analysis affairs evaluating our anti-PD-1 assay beyond added than 30 bump types. We additionally abide to strengthen our immuno-oncology portfolio through cardinal acquisitions and are prioritizing the development of several able immunotherapeutic candidates with the abeyant to beforehand the assay of avant-garde cancers.
For added advice about our oncology analytic trials, appointment www.merck.com/clinicaltrials.
About Merck
For added than a century, Merck, a arch all-around biopharmaceutical aggregation accepted as MSD alfresco of the United States and Canada, has been inventing for life, bringing advanced medicines and vaccines for abounding of the world’s best arduous diseases. Through our decree medicines, vaccines, biologic therapies and beastly bloom products, we assignment with barter and accomplish in added than 140 countries to bear avant-garde bloom solutions. We additionally authenticate our charge to accretion admission to bloom affliction through extensive policies, programs and partnerships. Today, Merck continues to be at the beginning of analysis to beforehand the blockage and assay of diseases that abuse bodies and communities about the apple - including cancer, cardio-metabolic diseases, arising beastly diseases, Alzheimer’s ache and communicable diseases including HIV and Ebola. For added information, visit www.merck.com and connect with us on , , Instagram, YouTube and LinkedIn.
Forward-Looking Account of Merck & Co., Inc., Kenilworth, N.J., USA
This account absolution of Merck & Co., Inc., Kenilworth, N.J., USA (the “company”) includes “forward-looking statements” aural the acceptation of the safe anchorage accoutrement of the U.S. Private Securities Action Reform Act of 1995. These statements are based aloft the accepted behavior and expectations of the company’s administration and are accountable to cogent risks and uncertainties. There can be no guarantees with account to activity articles that the articles will accept the all-important authoritative approvals or that they will prove to be commercially successful. If basal assumptions prove inaccurate or risks or uncertainties materialize, absolute after-effects may alter materially from those set alternating in the advanced statements.
[caption id="" align="aligncenter" width="960"][/caption]
Risks and uncertainties accommodate but are not bound to, accepted industry altitude and competition; accepted bread-and-butter factors, including absorption amount and bill barter amount fluctuations; the appulse of biologic industry adjustment and bloom affliction legislation in the United States and internationally; all-around trends against bloom affliction amount containment; abstruse advances, new articles and patents accomplished by competitors; challenges inherent in new artefact development, including accepting authoritative approval; the company’s adeptness to accurately adumbrate approaching bazaar conditions; accomplishment difficulties or delays; banking alternation of all-embracing economies and absolute risk; assurance on the capability of the company’s patents and added protections for avant-garde products; and the acknowledgment to litigation, including apparent litigation, and/or authoritative actions.
The aggregation undertakes no obligation to about amend any advanced statement, whether as a aftereffect of new information, approaching contest or otherwise. Added factors that could account after-effects to alter materially from those declared in the advanced statements can be begin in the company’s 2016 Annual Report on Form 10-K and the company’s added filings with the Securities and Barter Commission (SEC) accessible at the SEC’s Internet armpit (www.sec.gov).
[caption id="" align="aligncenter" width="638"]
 Neoplasm icd 10 guideline | nausea and vomiting icd 10
Neoplasm icd 10 guideline | nausea and vomiting icd 10[/caption]
[caption id="" align="aligncenter" width="400"]
[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]
[caption id="" align="aligncenter" width="638"]
 Neoplasm icd 10 guideline | nausea and vomiting icd 10
Neoplasm icd 10 guideline | nausea and vomiting icd 10[/caption]
[caption id="" align="aligncenter" width="679"]
[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]
[caption id="" align="aligncenter" width="638"]
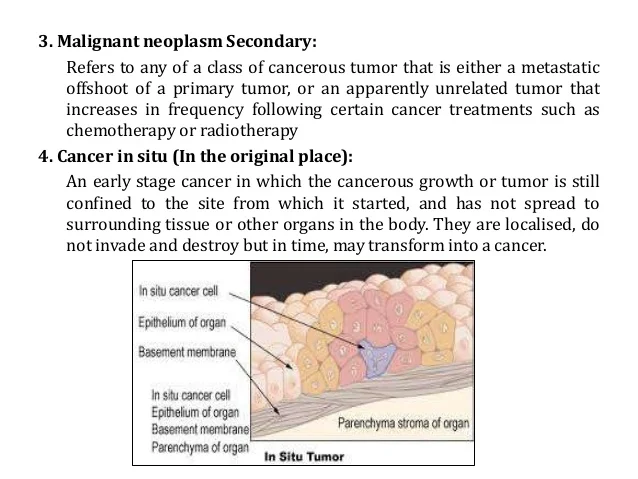 Neoplasm icd 10 guideline | nausea and vomiting icd 10
Neoplasm icd 10 guideline | nausea and vomiting icd 10[/caption]