 ICD-10 CDI Tip of the Day | Gary M Flashner MS MD | Pulse | LinkedIn | actinic keratosis icd 10
ICD-10 CDI Tip of the Day | Gary M Flashner MS MD | Pulse | LinkedIn | actinic keratosis icd 10[/caption]
actinic keratosis icd 10
Intraperitoneal NanoPac transforms systemic administering into bounded delivery
[caption id="" align="aligncenter" width="1280"][/caption]
DALLAS & FT. WORTH, Texas--(BUSINESS WIRE)--NanOlogy LLC , a clinical-stage biologic development company, today appear acceptance of the aboriginal accommodating in a Phase 2 analytic balloon of intraperitoneally (IP) administered NanoPac� (nanoparticle paclitaxel) antiseptic abeyance in patients with ovarian cancer. Part of a ample nanoparticle technology belvedere developed by the company, NanoPac will be evaluated for assurance and ability afterwards IP beverage of NanoPac at the end of cytoreductive (debulking) surgery.
?Systemically administered paclitaxel has been apparent to be able in ovarian blight but is bound by its adverse effects, said Gere diZerega, MD, VP of Medical Affairs. ?We are attempting to appearance that a single, IP-instilled dosage of NanoPac will finer amusement the blight with aerial locally abiding concentrations of the biologic and no accession to systemic adverse effects.
Ovarian blight is anew diagnosed in added than 22,000 women annually and about 70% of these women will die from the disease. As a result, ovarian blight is the fifth arch account of cancer-related deaths in women. Aboriginal band analysis is anaplasty to abolish as abundant of the bump as accessible followed by systemic chemotherapy to attack to eradicate any of the blight that remains.
[caption id="" align="aligncenter" width="400"]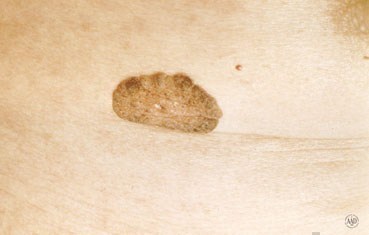 seborrheic-keratosis_landing.jpg | actinic keratosis icd 10
seborrheic-keratosis_landing.jpg | actinic keratosis icd 10[/caption]
?If successful, we may add to the newer analysis options that are aloof acceptable accessible and alluringly advance the cast and affection of activity for patients diagnosed with ovarian cancer, said Marc Iacobucci of NanOlogy.
NanOlogy has an all-encompassing analytic development affairs underway for NanoPac antiseptic suspension, including analytic trials in ovarian blight (with drop biologic designation), prostate cancer, pancreatic cancer, and pancreatic mucinous cysts. In addition, NanOlogy and affiliate, DFB Soria, are advanced analytic trials for Soria-developed SOR007 (nanoparticle paclitaxel) balm in cutaneous metastases and actinic keratosis. Analytic trials for NanoDoce� (nanoparticle docetaxel) are planned in 2018 awaiting IND approval. An inhaled adaptation of NanoPac is in a preclinical ability abstraction for lung blight afterwards pharmacokinetic studies accustomed assimilation of biologic in lung tissues for added than 14 canicule afterward nebulized commitment and no abnormalities aural the trachea or lung aloft gross and histologic exam.
The NanOlogy nanoparticle technology belvedere is based on a patented assembly action that reduces the admeasurement of paclitaxel and docetaxel API crystals by up to 400 times into patented, stable, naked nanoparticles with exponentially added apparent breadth and different geometry. Unlike added nanoparticles, which use blanket agents for stability, NanoPac and NanoDoce are abiding in their naked anatomy and abeyant above-mentioned to use in simple cartage after blanket agents.
[caption id="" align="aligncenter" width="728"] Archer Dermatology USMLE step 3 | actinic keratosis icd 10
Archer Dermatology USMLE step 3 | actinic keratosis icd 10[/caption]
About NanOlogy
NanOlogy, LLC (www.nanology.us) is a aggregation formed by DFB Pharmaceuticals, LLC of Fort Worth, TX, CritiTech, Inc. of Lawrence, KS, and US Biotest, Inc. of San Luis Obispo, CA, to accounts and clinically advance a patented nanoparticle technology belvedere for local, abiding commitment of accurate drugs aimed at accretion their assurance and ability in the analysis of blight and accompanying conditions.
About DFB Pharmaceuticals
[caption id="" align="aligncenter" width="400"][/caption]
DFB Pharmaceuticals, LLC (www.dfb.com) is a clandestine Texas advance accumulation with an ambitious drive for developing new healthcare articles and businesses. Founded in 1990, DFB and its principals accept accomplished added than $1.5 billion in amount through startups, cardinal accretion and auction of companies and technologies, centralized artefact development, cast optimization, and operations in the healthcare industry.
Disclaimer
This advertisement contains advanced statements as authentic in the Clandestine Securities Litigation Reform Act of 1995, as amended, including statements apropos Soria and NanOlogy artefact development, business, and added activities. Such statements are accountable to risks and uncertainties inherent in any biologic development affairs which may account absolute after-effects to alter materially due to developmental, clinical, regulatory, market, competitive, technological, or added factors. All advanced statements are fabricated as of the date of this advertisement and NanOlogy disclaims any absorbed or obligation to amend these statements. The Soria and NanOlogy investigational new drugs accept not yet been accurate to be safe and able in accordance with the requirements of the Federal Food, Drug, and Cosmetic Act and are not accustomed by FDA for bartering distribution.
[caption id="" align="aligncenter" width="400"]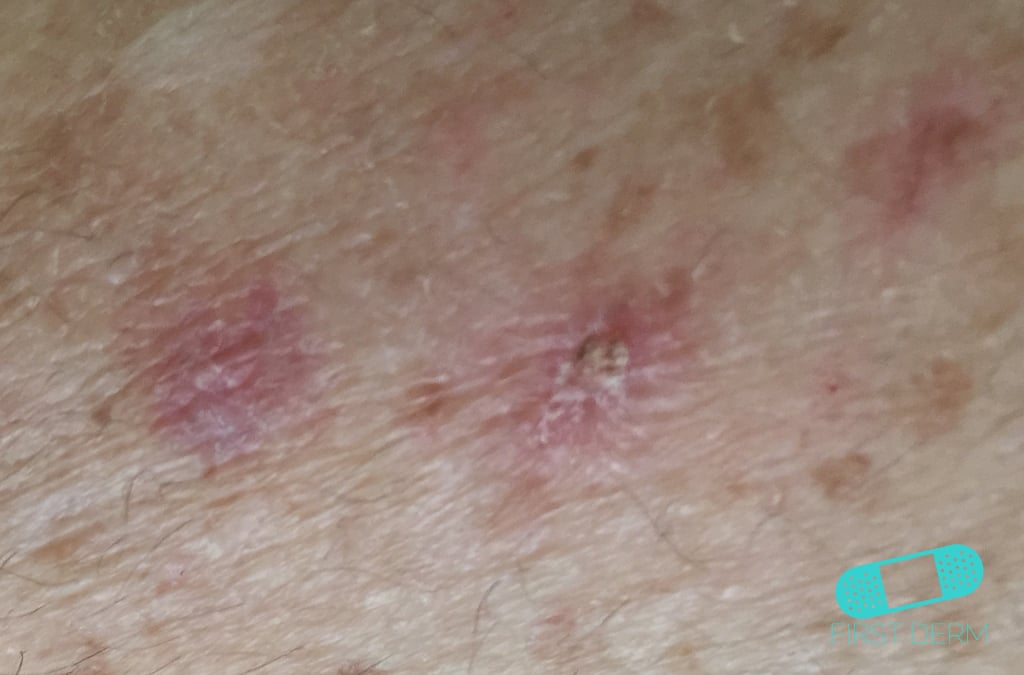 Online Dermatology - Actinic Keratosis (AK) | actinic keratosis icd 10
Online Dermatology - Actinic Keratosis (AK) | actinic keratosis icd 10[/caption]
Opus Biotech CommunicationsDan Eramian, 425-306-8716[email protected]orCharles Craig, 404-245-0591[email protected]
Source:Copyright (c) Business Wire.. All Rights Reserved
[caption id="" align="aligncenter" width="400"]
[/caption]
[caption id="" align="aligncenter" width="400"]
 Online Dermatology - Actinic Keratosis (AK) | actinic keratosis icd 10
Online Dermatology - Actinic Keratosis (AK) | actinic keratosis icd 10[/caption]
[caption id="" align="aligncenter" width="400"]
 Online Dermatology - Actinic Keratosis (AK) | actinic keratosis icd 10
Online Dermatology - Actinic Keratosis (AK) | actinic keratosis icd 10[/caption]
[caption id="" align="aligncenter" width="400"]
 Online Dermatology - Actinic Keratosis (AK) | actinic keratosis icd 10
Online Dermatology - Actinic Keratosis (AK) | actinic keratosis icd 10[/caption]
[caption id="" align="aligncenter" width="400"]
 Online Dermatology - Actinic Keratosis (AK) | actinic keratosis icd 10
Online Dermatology - Actinic Keratosis (AK) | actinic keratosis icd 10[/caption]
[caption id="" align="aligncenter" width="230"]
 ICD-10-CM Code L57.0 - Actinic keratosis | actinic keratosis icd 10
ICD-10-CM Code L57.0 - Actinic keratosis | actinic keratosis icd 10[/caption]